Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Medicinal Chemistry
Engineered enzymes help remix natural products
Researchers transform a single precursor molecule into 51 new and diverse structures
by Brianna Barbu
September 11, 2024
Nature is the undisputed champion of creating complex organic molecules, many of which humans have used for many years to create medicines. Chemists have spent over a century honing their ability to make molecules that mimic certain properties of natural products and tinkering with natural products to fine-tune their function.
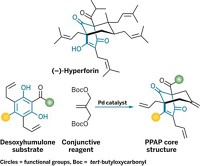
A team of chemists led by Rudi Fasan of the University of Texas at Dallas have now taken riffing on natural products to a whole new level (Chem 2024, DOI: 10.1016/j.chempr.2024.08.003). The researchers took parthenolide, a component of the medicinal herb feverfew, and remixed the molecule’s carbon skeleton using engineered enzymes and additional chemical modifications to create 51 completely new compounds with high structural diversity and intriguing anticancer properties.

“Instead of making analogs of the parent molecule, we can create entirely new scaffolds,” Fasan says, adding that this is one of his favorite projects to have come out of his lab.
Parthenolide’s biological activity arises from an α-methylene-γ-lactone “warhead” that covalently binds to target proteins. The researchers aimed to keep the lactone intact while playing with the rest of the carbon skeleton. They used three different engineered P450 enzymes to carry out selective C–H oxidations and epoxidations. Fasan says those enzymatic transformations provided a “springboard” to additional structural rejiggering using more traditional chemistries such as ring-closing metathesis or Diels-Alder cycloadditions.

In many cases they could predict the products that would result from a particular sequence of transformations, but there were some surprise rearrangements, Fasan says.
All but one of the new molecules had higher molecular diversity than the original parthenolide, and all scored well on the “rule of five” properties that medicinal chemists often look for. ”They’re all druglike,” Fasan says.
The researchers tested the compounds against eight human cancer cell lines and found that several molecules had promising anticancer activity. Some of them worked against multiple types of cancer, while others were more selective. Fasan says these molecules could be potential drug leads or could work as chemical probes to discover new protein targets and pathways to target for cancer drug development.
Robert Huigens III, a medicinal chemist at the University of Georgia who was not involved with the work, called it “elegant, creative, strategic and innovative . . . a must read for anyone interested in chemical biology and drug discovery.” Huigens says he’s excited to see more work to develop the cancer-targeting properties of these new molecules.
Fasan says his group’s next steps are to follow up on the new molecules they have made and to extend the strategy to more enzymes and parent molecules to continue exploring new natural product–like structures.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter