Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Reagents
Sustainable organosodium compounds step up in cross-coupling reactions
A low-concentration sodium dispersion allows chemists to make organosodium reagents from cheap aryl chloride precursors
by Tien Nguyen
March 21, 2019
| A version of this story appeared in
Volume 97, Issue 12

Organolithium and magnesium reagents are staples in chemical stockrooms. Useful in an array of reactions, they may experience their heaviest demand from cross-coupling reactions. In two such reactions, the Nobel Prize–winning Suzuki and Negishi cross couplings, a transition-metal catalyst helps boron or zinc compounds—which are made from lithium or magnesium reagents—form C–C bonds with other molecular partners. Chemists make these reagents by mixing the metals with aryl bromides and iodides, and sometimes, with less reactive and less expensive aryl chlorides, but that process requires harsher conditions.
Now, researchers at Okayama University report a mild way to activate cheap aryl chlorides using sodium, a metal that has long been overshadowed by its more popular neighbors on the periodic table (Nat. Catal. 2019, DOI: 10.1038/s41929-019-0250-6).
Although it’s nontoxic and the most abundant alkali metal in the earth’s crust, chemists have historically shied away from sodium because of its extreme reactivity. The metal catches fire upon the slightest contact with water, posing serious safety as well as handling issues. For example, commercially available sodium lumps come submerged in oil, so to use it, researchers must weigh out carved-off slivers under an inert atmosphere.
The authors’ method addresses sodium’s safety issues by using a low-concentration dispersion of the metal in oil (26 wt %). At this concentration, the authors say, sodium is stable and chemists can weigh it out in air with a syringe. “But still, we have to carefully handle the sodium dispersion because it’s sodium after all.”
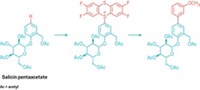
The company Kobelco Eco-Solutions originally produced this dispersion to help dispose of chlorinated organic pollutants by breaking down the molecules. The company reached out to the Okayama team in hopes of finding a use for the mixture in organic synthesis. The researchers found that treating aryl chlorides with the sodium dispersion at 30 °C generated organosodium reagents that could be used in a number of Negishi, Suzuki, and direct Pd-catalyzed coupling reactions (shown).
“This work is another step in the direction of using more earth-abundant metals in organic synthesis,” says Sharon R. Neufeldt, an organic chemist at Montana State University. While chemists usually think about the sustainability of the transition-metal catalyst in a reaction, she says, these results highlight the need to consider the stoichiometric organometallic reagents as well.
The team says they’re working on expanding the scope of organosodium reagents and exploring their use in other ubiquitous transition-metal-catalyzed reactions.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter