Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
ACS Meeting News
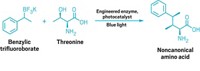
Engineered enzyme flirts with fluoroalkylation
Light-activated enzyme attaches fluorinated groups to molecules with high selectivity
by Brianna Barbu
August 19, 2024

There are many chemical methods for adding medicinally helpful fluorinated alkyl groups onto molecules, but doing so with high stereoselectivity can be a challenge, especially at remote positions. Enzymes, meanwhile, are superstars of stereoselectivity—but there’s only one enzyme that naturally works with fluorinated substrates. Fortunately, it’s possible to teach old enzymes new tricks.
Researchers led by Huimin Zhao of the University of Illinois at Urbana-Champaign have engineered a light-activatedenzyme to stereoselectively attach fluorinated alkyl groups to molecules that contain double bonds. They recently published a paper about the work in Science (2024, DOI: 10.1126/science.adk8464). Postdoctoral fellow Maolin Li, the paper’s first author, presented the work on Sunday at ACS Fall 2024 to the Division of Organic Chemistry.
Zhao says that his group engineers enzymes with the goal of bringing together the versatility of radical photocatalysis and the selectivity of biocatalysis. Their enzymes of choice in this pursuit come from a family of ene reductases called old yellow enzymes, which uses a light-driven radical mechanism to facilitate stereoselective addition reactions to double bonds. The group had previously engineered enzymes to make chiral amines, carbonyls, and more before they arrived at fluoroalkylation.
Li said that he had initially expected that the reaction would work best with a fluorination source that contained an oxygen-containing leaving group that could bind into the enzyme’s active site. “We tried a lot of fluorine reagents, but all of them failed,” he says, until he switched to fluorinated alkyl iodides.
Having shown that the enzyme could indeed handle fluoroalkylation, the researchers deployed information gathered from molecular docking simulations to make strategic edits to the protein sequence. This helped boost both the yield and enantioselectivity. Depending on the fluorinated starting material used, the enzymatic reaction can generate products with stereocenters up to four carbon atoms away from the nearest fluorine atom.
Yang Yang of the University of California, Santa Barbara, a photoenzyme expert who was not involved with the study, calls the paper “an exciting example” of how enzymes can be repurposed to accomplish challenging reactions.
Zhao and Li say that they plan to work on boosting their fluoroalkylating enzyme’s activity further so that it can handle larger-scale reactions more efficiently. And, of course, they want to continue developing more new light-activated enzymes to accomplish more challenging reactions.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter