Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
ACS Meeting News
Scientists reveal diverse compounds at ACS’s ‘First Disclosures’ session
Chemists presented drug candidates to treat diseases from Crohn’s to cancer
by Leigh Krietsch Boerner
April 6, 2023
| A version of this story appeared in
Volume 101, Issue 12
At most American Chemical Society meetings, the “First Time Disclosures” of clinical candidates session packs in medicinal chemists curious about what drug companies are working on. This ACS Spring 2023 meeting was no different. Scientists from the companies Nurix Therapeutics, AbbVie, Merck & Co., and Boehringer Ingelheim presented their drug candidates’ results from clinical trials. Session organizer Nicole Goodwin from GSK commented on how the candidates presented were all very different from one another this year. Goodwin pointed out in her introduction that this particular session was a great example of how the field has evolved to take on the most interesting and difficult targets with chemistry.
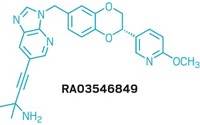
Candidate: NX-2127
Presenter: Jeffrey Wu, Nurix Therapeutics
Target: Bruton’s tyrosine kinase
Disease: B-cell malignancies

Jeffrey Wu from Nurix Therapeutics presented the clinical candidate NX-2127, an oral medication that degrades Bruton’s tyrosine kinase (BTK). This enzyme is involved in the signaling pathway that allows leukemia cells to survive in cancers that affect a type of white blood cell called B cells. Scientists have found that small-molecule BTK inhibitors work to destroy B-cell tumors, and the US Food and Drug Administration has approved these inhibitors. But some types of B-cell tumors have developed resistance to these drugs. To get around this, Nurix’s clinical candidate NX-2127 combines the activity of a targeted BTK degrader with a second compound that degrades the Ikaros zinc finger transcription factors, which are responsible for cell differentiation in B cells.
Wu and coworkers found that joining these two compounds together via linkers of different sizes was effective against tumors, including in people that had BTK-inhibitor-resistant mutations. In the multiple variations of linked compounds, the researchers found that differences in potency were due to the sizes of molecular rings in the linkers. In addition, lowering the molecular weight improved pharmacodynamic properties in mice. NX-2127 was more potent and had broader activity than all the other compounds the group tested, and it was highly bioavailable in mouse studies, Wu said. In clinical studies, the drug degraded 80% of people’s BTK, including in those that had mutations. The compound is currently in Phase 1b clinical trials.
Candidate: ABBV-154
Presenter: Adrian Hobson, AbbVie
Target: Tumor necrosis factor
Disease: Crohn’s disease and polymyalgia rheumatica

Adrian Hobson from AbbVie spoke about ABBV-154, a compound to treat inflammatory diseases such as Crohn’s disease and polymyalgia rheumatica by inhibiting the tumor necrosis factor. White blood cells make the tumor necrosis factor proteins in response to an inflammation or an antigen in the body. AbbVie’s compound connects an antibody to an immunology antibody-drug conjugate (ADC) using a glucocorticoid receptor modulator (GRM) as a linker. Scientists have used GRMs in the past to treat inflammatory diseases because the compounds promote the release of anti-inflammatory proteins in the body. But at doses high enough to be effective, GRMs have many unwanted side effects. To get around this, Hobson and team attached the GRM to an ADC. Their system can deliver the drug candidate directly to the target more efficiently than the GRM can get there by itself, cutting the dose needed to treat the disease. This means there would be fewer side effects in patients.
The team mined the literature to try to find a good candidate for the ADC, and it homed in on steroids. The compounds make good immunology drugs, but the problem is finding a stable place to attach the steroid to the payload, Hobson said. Otherwise, the active part of the compound might fall off in circulation, before it can get to the right cells. Ultimately, Hobson and coworkers added a two amino–acid sequence and included an aminobenzyl alcohol spacer, which can release the GRM at the right time. The drug is in Phase 2 clinical trials.
Candidate: MK-0616br
Presenter: Abbas Walji, Merck & Co.
Target: PCSK9
Disease: Heart disease

Low-density lipoprotein cholesterol (LDL) is the bad kind of cholesterol. High levels can increase the risk of heart attack and stroke. Doctors often prescribe drugs called statins to lower LDL, but this treatment does not work for some people, and it comes with a host of side effects. Abbas Walji from Merck & Co. shared the development of MK-0616, an oral macrocyclic peptide that inhibits PCSK9. PCSK9 is short for “proprotein convertase subtilisin/kexin type 9,” and it’s a protein made in the liver that destroys LDL receptors. Inhibiting this protein means that more LDL receptors are around to remove LDL from the blood, effectively lowering bad cholesterol. Other PCSK9 inhibitors have appeared on the drug market in the past and have shown promise, but the treatments have had issues, including high cost.
Walji and the team designed their macrocyclic peptide using structure-based drug design, he said in the session. They modeled the compound’s key interactions with the protein and found that a fluorotryptophan in the center was important to the drug’s activity and that their molecule had five hydrogen bonds with one of PCSK9’s β sheets. The team tweaked the molecule on the basis of these findings and tested it on monkeys and in human plasma. Walji said that the researchers saw an 80% reduction of unbound PCSK9, which corresponded to 50% lower LDL levels in human plasma after 14 days compared with treatment with statins. Further plasma studies showed a 60% reduction in LDL at doses of 10–20 mg. MK-0616 is in Phase 2b trials, and preliminary results are showing that LDL is reduced up to 60.9% after 8 weeks compared with levels in people given a placebo.
Candidate: BI 1015550
Presenter: Horst Dollinger, Boehringer Ingelheim
Target: Phosphodiesterase 4
Disease: Idiopathic pulmonary fibrosis
Horst Dollinger from Boehringer Ingelheim presented BI 1015550, which targets a phosphodiesterase 4 (PDE4) enzyme. The company is developing this compound to treat idiopathic pulmonary fibrosis (IPF), a progressive disease that thickens tissues in the lungs. There are currently two treatments approved by the US Food and Drug Administration for the irreversible lung condition, but these drugs can only slow the advancement, not stop it. In preclinical studies, inhibiting the PDE4B variety of the enzyme reduced inflammation and impeded the formation of fibrous tissue in the lungs.
Dollinger presented the results from Phase 2 trials of BI 1015550, both by itself and in combination with a known antifibroid drug. The team found that in a 12-week randomized, double-anonymized, placebo-controlled trial of people with IPF, BI 1015550 did not harm lung function, either by itself or with the antifibroid. Next up for the compound are two longer Phase 3 trials in people with IPF and other similar lung diseases.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter