Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Diagnostics
Firms aim to develop liquid biopsies for early cancer detection
Freenome, Grail, and others that are planning to bring the blood tests to market have their work cut out for them
by Jyoti Madhusoodanan, special to C&EN
December 1, 2019
| A version of this story appeared in
Volume 97, Issue 47

Muscles, bones, and organs become less elastic and more injury prone as we age. Our cells parallel this process, accumulating genetic mutations, changes to chemical markers on DNA, and other genetic blips. Collectively, these errors increase our odds of developing tumors as we get older: adults over the age of 50 have a cancer rate several times as high as the rate for adults in their twenties, according to data from the US National Cancer Institute.
What if there were a diagnostic that doctors could use to monitor these genetic blips as we age? The millions who develop cancers in their lungs, breasts, kidneys, and other tissues each year might be identified in the earliest stages of disease—that is, when treatments are most effective.
When tumors form, they aren’t completely stealthy about it. Many cancers—regardless of where they’re located in the body—shed cells, DNA, and other material into the bloodstream. And for years, researchers have attempted to pinpoint the identity and location of tumors by tracing these leaks back to their sources. The first tests for these types of so-called circulating tumor markers aimed to identify genetic hallmarks, such as mutations or gene expression changes, that would help develop targeted drugs. Companies such as Grail, Freenome, Thrive Earlier Detection, and others are now setting their sights on a greater goal: blood tests that can spot cancers long before symptoms appear.
As scientists have learned which biomarkers in our blood signal cancer rather than aging, and as they’ve learned which types of tumors shed biomarkers and why, advances in technology have brought such a blood test within reach. A test like this would arguably benefit people whose diseases might be caught early. But it would also be profitable for the firms that develop it: rather than being offered to already-diagnosed patients—a smaller population—a diagnostic that screens for multiple kinds of cancers from a single blood sample would ideally be offered to all older adults as part of an annual physical.
“Early detection is obviously a much, much larger market than testing patients who are known to have cancer,” says computational biologist Imran Haque, who leads data science at Recursion Pharmaceuticals. “We’re now seeing a lot of folks who are willing to invest money in developing technology to try to address the early-detection market.”
Early challenges
The earliest blood tests that detected DNA shed from bodily tissues were designed not for aging adults but for babies. These diagnostics look for fetal DNA anomalies like extra chromosomes that cause trisomies such as Down’s syndrome, or missing chunks of DNA in a sample of the mother’s blood. These tests are routinely administered to pregnant women. In 2015, though, researchers found that in addition to detecting fetal DNA mutations, these types of tests could spot tumor DNA from the mother in a sample of her blood (JAMA, J. Am. Med. Assoc. 2015, DOI: 10.1001/jama.2015.7120).
At the time, similar blood tests known as liquid biopsies were being developed to detect late-stage cancers in lung, breast, and other tissues, the idea being that if scientists could learn what genetic changes spurred a tumor to grow, they might be able to target them with drugs. For instance, pharma company Amgen recently developed the first inhibitor of KRAS, a long-pursued cancer target. With a blood test to detect KRAS mutations sensitive to this drug, clinicians could quickly find patients most likely to benefit from the treatment.
DNA shed by tumors into the bloodstream was also being used to monitor people diagnosed with cancer, to see how they were responding to treatments. But clinicians knew what cancers these patients had, and their tumors—which are larger, more numerous, or in advanced stages—shed more biomarkers, making them easier to spot in a blood sample.
Early detection, however, is much more challenging. It focuses on people with no apparent tumors—just a risk of developing them. Some firms, such as San Carlos, California–based Natera, are developing liquid biopsies for people who have successfully had a tumor excised and need additional monitoring. Others aim to create tests for anyone in the general population who may develop a cancer. Both groups face gargantuan obstacles: a successful test must be highly sensitive, meaning it correctly spots signs of cancer, as well as highly specific, meaning it doesn’t identify a tumor when there isn’t one.
In the earliest stages of cancer, only about 1 in 10,000 pieces of free DNA floating in the blood will be from a tumor. “A typical 10 mL tube of blood contains something like 10,000 single copies of DNA,” says Alexey Aleshin, Natera’s oncology medical director. “So just by chance alone, you may draw blood, and that one molecule won’t be in that particular tube. Even if you have a perfect test, you can’t detect something that’s not there.”
So Natera’s Signatera test uses a tumor-informed approach, in which tissue that was previously excised from a person with cancer is sequenced to identify its unique genetic signature. Then, a bespoke test is designed to spot that fingerprint going forward. In studies of people who have had surgery for bladder, lung, breast, or colorectal cancer, the firm has found its assay can detect recurrence several months earlier than imaging tests (J. Clin. Oncol. 2019, DOI: 10.1200/JCO.18.02052). But this tumor-informed approach isn’t feasible for screening someone who’s never been diagnosed.
Another challenge, Aleshin points out, is that age-related, random mutations grow increasingly common in older adults—who are at the greatest risk of cancer—and these mutations are often indistinguishable from those that spur tumors. “If you don’t know what you’re looking for and you find these mutations, the first question that comes up is whether they’re cancer related or just biological noise,” Aleshin says.

Bountiful biomarkers
Instead of seeking tumor-specific signatures, liquid biopsies that take a tumor-blind approach rely on technological advances—and a better understanding of cancer itself—for success.
Most of these early-detection tests have expanded beyond looking for mutations on circulating DNA to include panels of chemical tags on DNA, such as methyl groups, known as epigenetic markers. They also look at the sizes of DNA fragments released from tumors. New versions of diagnostics rely on machine-learning algorithms trained on large data sets to distinguish tumor-related signatures from non-tumor-related ones. The additional biomarkers and the algorithms aim to boost both sensitivity and specificity.
Including epigenetic and other signals also circumvents the noise of random, noncancerous mutations that crop up in the genome as we age, says Alex Aravanis, head of R&D at Menlo Park, California–based Grail. “While mutations are useful for therapy selection, they tend to be problematic for early detection,” he says. “Methylation patterns are more sensitive to most cancer types.”
In 2016, Grail launched its Circulating Cell-Free Genome Atlas (CCGA) Study, in which researchers screened 15,000 people over the age of 50 for genomic changes over time. Approximately two-thirds of the participants had cancer; the rest were cancer-free at the start of the study. Five participants in the latter group tested positive for many cancer-like signals, and two went on to develop ovarian and endometrial cancers a few months after being given Grail’s blood test. The team tested combinations of markers such as DNA mutations and methylation but found that “using methylation alone provided a very specific signature that could identify more than 20 cancer types with 90% accuracy,” Aravanis says.
Cambridge, Massachusetts–based Thrive Earlier Detection aims to combine two biomarkers—genetic mutations and cancer-associated proteins—in its test, which is based on CancerSEEK, a diagnostic developed by researchers at Johns Hopkins University. In a 2018 study of 1,000 people with cancer and 850 healthy participants, the Johns Hopkins team reported that its blood test detected eight cancer types with a sensitivity of 69–98%. Only 7 of the 850 healthy individuals tested positive. Whether these people had as-yet-undetected cancers isn’t known, but even if they were all false positives, the test was incorrect in only about 1% of cases, meaning it had a specificity greater than 99% (Science 2018, DOI: 10.1126/science.aar3247).
Similar to these other companies, South San Francisco–based Freenome has developed a test that tracks DNA in the blood. But the firm is looking beyond cancer cells. “Quite simply, there’s just not enough DNA floating around in your blood from tumor cells for early detection to work,” says Gabriel Otte, cofounder of Freenome.
Freenome’s test looks for tumor DNA as well as free-floating DNA from immune cells that are involved in recognizing and attacking tumor-specific proteins. “Many more immune cells are attacking the tumor and turning over than the number of tumor cells that are being shed and replaced in the early stages of cancer,” Otte says. “So the immune markers create an amplified signature that’s easier to detect.”
It’s currently impossible to compare the varied approaches of these early-detection companies head to head, says cancer researcher Maximilian Diehn of Stanford University. For starters, no epigenetics-based tests are currently used in the clinic, so whether they will meet the standards of mutation-based tests, like the early ones used to develop targeted cancer drugs, is still to be determined. Moreover, each company has tested its assays in separate studies with independent groups of participants—and even little differences in individuals’ disease states could affect the reported results.
For instance, Diehn says, two people with stage 1 cancers—one with fewer, large tumors and another with numerous, smaller tumors—might have different amounts of circulating biomarkers. “A difference like that in tumor burden between cohorts could make a big difference to how sensitive a test seems to be,” he says. “There’s a natural tendency to want to compare the assays, but that’s a risky business.”
The contenders
The firms developing liquid biopsies for early cancer detection are all sampling different biomarkers in blood.
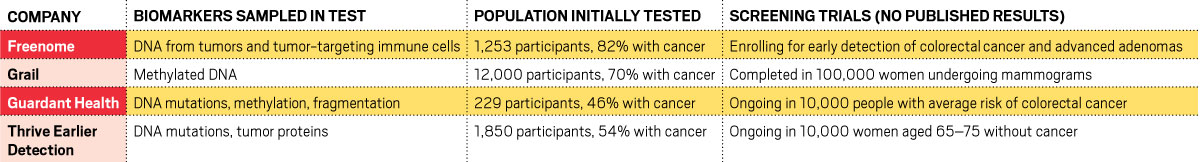
Panning for positives
All these players have launched or recently completed prospective clinical trials in older adults, with results from many expected in 2020. Thrive, for instance, has collaborated with Pennsylvania’s Geisinger Health system to screen 10,000 women, aged 65–75, with no history of cancer. Participants will be tested for 16 genetic mutations and 11 proteins linked to cancers.
Freenome and others also have similar trials underway in participants with no history of cancer. So far, none has reported results from these large-scale prospective studies. The studies are slow, expensive endeavors: even among 55-to-70-year-old adults, who are at a much higher risk of cancer than younger people, only 800 in every 100,000 individuals were diagnosed with cancer in 2019. A screening test to catch that small percentage of cases in the early stages of disease needs to be tested on several thousand people who must be followed over time before a company can confirm that its test works with the requisite sensitivity and specificity.
Advertisement
Along the long road to the clinic, firms offering such tests must also grapple with what the demand will be for their diagnostics, should they be successful. For some cancers, the benefits of early detection have been demonstrated. Screening can catch tumors in the early stages of colorectal cancer, for instance, and the tumors can be excised in a colonoscopy, leading to a higher survival rate than without early detection. But that’s not true for all cancers. Some are so rare that a population-wide screening isn’t justified; others are slow-growing forms that may not require immediate clinical care. For others, such as ovarian cancer, guidelines from the US Preventive Services Task Force recommend against early screening because catching the disease early offers no benefits in terms of reducing mortality and instead increases the odds of substantial harm, including unnecessary surgery (JAMA, J. Am. Med. Assoc. 2018, DOI: 10.1001/jama.2017.21926).
“There’s a really high bar to show survival benefits,” Diehn says. “These large, randomized studies need to prove not just that more cancers were diagnosed in patients who got a screening test but that the health and survival of the entire group was better as a result of being screened.”
Jyoti Madhusoodanan is a freelance science writer based in Portland, Oregon.
CORRECTION
This story was updated on Dec. 9, 2019, to correct the statement from Grail head of R&D, Alex Aravanis, that Grail's blood test can identify more than 20 cancer types with 99% accuracy using DNA methylation markers. It can identify more than 20 cancer types with 90% accuracy using those markers.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter