Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.

Jose Rodriguez
Structural savant is imaging proteins that clump to better understand diseases such as Alzheimer’s
by Michael Torrice
August 20, 2018 | APPEARED IN VOLUME 96, ISSUE 33
Jose Rodriguez picked up structural biology first as a hobby. In his free time while working on a Ph.D. in molecular biology at the University of California, Los Angeles, he collaborated with a physics professor on a problem critical to how biologists process X-ray crystallography data.
Now, as a professor at UCLA, Rodriguez is using a relatively new structural biology technique to investigate proteins that clump together. These aggregating proteins are associated with diseases such as Alzheimer’s, and the UCLA scientist hopes that understanding their atomic-level details will pave the way to treatments.
“In cancer and metabolic diseases, there is a long, successful history of using structural information to design inhibitors that eventually become drugs,” says David Eisenberg of UCLA, who was Rodriguez’s postdoctoral adviser and is a current collaborator. But with these aggregating protein diseases, “there have been few structures so far, and that is the frontier that Jose has been pressing out.”
The technique Rodriguez uses is microelectron diffraction, or microED. In X-ray crystallography, scientists shine a beam of photons on a protein crystal, and its atoms scatter the photons in a characteristic way that can be analyzed to determine its structure. But conventional X-ray crystallography has trouble with crystals made from clumped proteins—they’re too small to produce meaningful data. MicroED, meanwhile, can yield high-resolution data from these crystals because it uses a beam of electrons that interacts more strongly with a crystal’s atoms than photons do.
In 2015, as a postdoc in Eisenberg’s lab, Rodriguez published the microED structure of α-synuclein, a protein that produces ropelike fibrils in the brains of people with Parkinson’s disease. It was the first structure of a protein obtained by microED that hadn’t been solved previously by another technique.
As an independent researcher, Rodriguez is studying prions, the infectious proteins responsible for diseases such as bovine spongiform encephalopathy, also known as mad cow disease. He hopes his structural insights could help explain prion mysteries such as how healthy proteins get converted into infectious ones that aggregate.
Rodriguez is also working to make microED accessible to a wide range of researchers. “It’s fine if I can do it,” he says, “but how do you make it easier to use so everyone can be a structural biologist?”
Watch Rodriguez speak at the American Chemical Society national meeting on Aug. 20 in Boston.
Vitals
Current affiliation: UCLA
Age: 32
Ph.D. alma mater: UCLA
Role model: Marie Curie. She was one of the best in an era of extraordinary scientists.
I’ve overcome adversity in the lab by: Once, an 11-day experiment failed with two days left to collect data. My team and I stayed up some unknown number of hours to recover what we could. The resulting data set saved the experiment and led to publication.
Latest TV show binge-watched: World Cup
Walk-up song: “Diablo rojo” by Rodrigo y Gabriela
Research at a glance
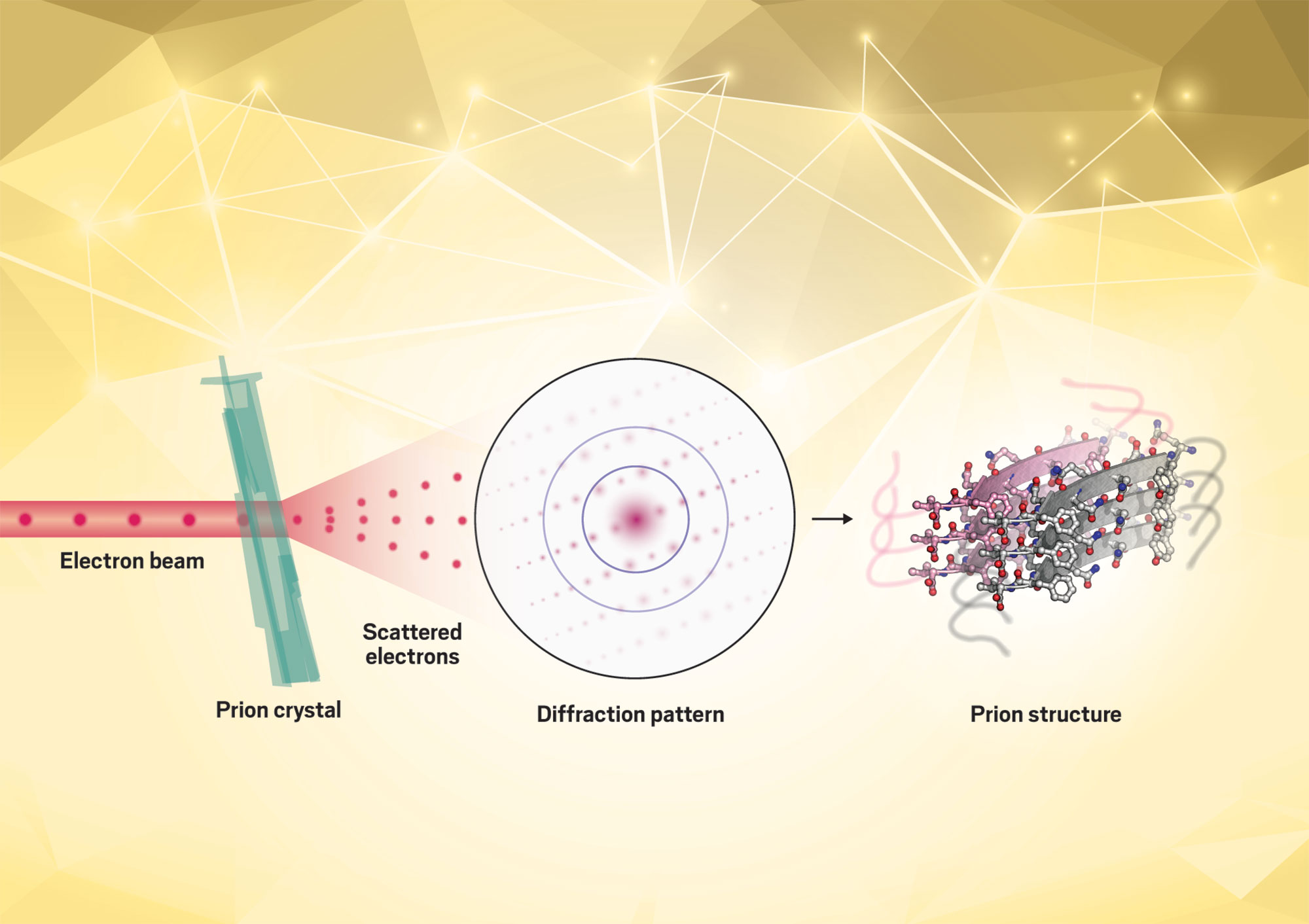
Credit: Jose Rodriguez/Yang H. Ku/C&EN/Shutterstock
To study the structures of clumping proteins using microelectron diffraction, Rodriguez aims a beam of electrons at the tiny protein crystals. By observing the pattern created by those scattering electrons, he can determine how individual proteins fold and stack onto each other.
Three key papers
"Sub-ångström Cryo-EM Structure of a Prion Protofibril Reveals a Polar Clasp"
(Nat. Struct. Mol. Biol. 2018, DOI: 10.1038/s41594-017-0018-0)
"Ab Initio Structure Determination from Prion Nanocrystals at Atomic Resolution by MicroED"
(Proc. Natl. Acad. Sci. USA 2016, DOI: 10.1073/pnas.1606287113)
Structural Biology
Jose Rodriguez
Structural savant is imaging proteins that clump to better understand diseases such as Alzheimer’s
by Michael Torrice
August 19, 2018
| A version of this story appeared in
Volume 96, Issue 33


COVER STORY
Jose Rodriguez
Vitals
Current affiliation: UCLA
Age: 32
Ph.D. alma mater: UCLA
Role model: Marie Curie. She was one of the best in an era of extraordinary scientists.
I’ve overcome adversity in the lab by: Once, an 11-day experiment failed with two days left to collect data. My team and I stayed up some unknown number of hours to recover what we could. The resulting data set saved the experiment and led to publication.
Latest TV show binge-watched: World Cup
Walk-up song: “Diablo rojo” by Rodrigo y Gabriela
Three key papers
“Sub-ångström Cryo-EM Structure of a Prion Protofibril Reveals a Polar Clasp” (Nat. Struct. Mol. Biol. 2018, DOI: 10.1038/s41594-017-0018-0)
“Ab Initio Structure Determination from Prion Nanocrystals at Atomic Resolution by MicroED” (Proc. Natl. Acad. Sci. USA 2016, DOI: 10.1073/pnas.1606287113)
“Structure of the Toxic Core of α-Synuclein from Invisible Crystals” (Nature 2015, DOI: 10.1038/nature15368)
Jose Rodriguez picked up structural biology first as a hobby. In his free time while working on a Ph.D. in molecular biology at the University of California, Los Angeles, he collaborated with a physics professor on a problem critical to how biologists process X-ray crystallography data.
Now, as a professor at UCLA, Rodriguez is using a relatively new structural biology technique to investigate proteins that clump together. These aggregating proteins are associated with diseases such as Alzheimer’s, and the UCLA scientist hopes that understanding their atomic-level details will pave the way to treatments.
“In cancer and metabolic diseases, there is a long, successful history of using structural information to design inhibitors that eventually become drugs,” says David Eisenberg of UCLA, who was Rodriguez’s postdoctoral adviser and is a current collaborator. But with these aggregating protein diseases, “there have been few structures so far, and that is the frontier that Jose has been pressing out.”
The technique Rodriguez uses is microelectron diffraction, or microED. In X-ray crystallography, scientists shine a beam of photons on a protein crystal, and its atoms scatter the photons in a characteristic way that can be analyzed to determine its structure. But conventional X-ray crystallography has trouble with crystals made from clumped proteins—they’re too small to produce meaningful data. MicroED, meanwhile, can yield high-resolution data from these crystals because it uses a beam of electrons that interacts more strongly with a crystal’s atoms than photons do.
In 2015, as a postdoc in Eisenberg’s lab, Rodriguez published the microED structure of α-synuclein, a protein that produces ropelike fibrils in the brains of people with Parkinson’s disease. It was the first structure of a protein obtained by microED that hadn’t been solved previously by another technique.
As an independent researcher, Rodriguez is studying prions, the infectious proteins responsible for diseases such as bovine spongiform encephalopathy, also known as mad cow disease. He hopes his structural insights could help explain prion mysteries such as how healthy proteins get converted into infectious ones that aggregate.
Rodriguez is also working to make microED accessible to a wide range of researchers. “It’s fine if I can do it,” he says, “but how do you make it easier to use so everyone can be a structural biologist?”.
















Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter