Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Inhibitors Target Key TB Enzyme
Iminosugars may provide leads for new class of tuberculosis drugs
by MICHAEL FREEMANTLE
September 6, 2004
| A version of this story appeared in
Volume 82, Issue 36

DRUG DISCOVERY
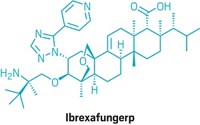
Scientists in England have designed and synthesized the first inhibitors of an enzyme that is essential for the survival of the tuberculosis bacterium [Org. Biomol. Chem., 2, 2418 (2004)]. The compounds might lead to better treatments for TB, which annually infects 8 million to 10 million people and kills 2 million to 3 million.
Mycobacterium tuberculosis can survive in humans for years under the protection of a waxy outer coat of its cell wall. Researchers hope that the new compounds will disrupt production of that coating and kill the bacterium.
"Our paper describes inhibitors of a transferase enzyme for which no previous inhibitors have been reported," says University of Nottingham chemist Neil R. Thomas, who carried out the research with coworkers there and at the University of Birmingham.
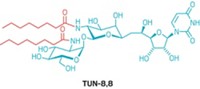
The compounds, two iminosugars, inhibit the biosynthesis of polysaccharides known as galactans. The main constituents of the galactans, galactofuranose residues, are not encountered in mammalian metabolism. Therefore, galactan biosynthesis constitutes an attractive target for potential new TB drugs lacking deleterious side effects, the authors observe.
The biosynthesis involves two enzymes. One, uridine diphosphate galactopyranose (UDP-Galp) mutase, catalyzes the conversion of UDP-Galp to its furanose counterpart UDP-Galf. The other, UDP-Galp transferase, catalyzes the transfer of UDP-Galf onto a growing oligosaccharide chain.
"Disrupting the production of the cell wall is known to kill the bacteria, and this is how current drugs work," Thomas explains. "However, the bacteria are rapidly developing resistance to established therapies, and the World Health Organization has a target to get one new drug on the market by 2007 and another by 2012."
Although the two iminosugars exhibit only moderate inhibitory activity against mycobacterial galactan biosynthesis, Thomas and coworkers hope that their compounds will provide a starting point for the development of more potent transferase inhibitors that could be considered for clinical trials.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter