Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Channel Protein Steers Gas
X-ray structure reveals how protein ushers NH3 across cell membranes
by Amanda Yarnell
September 13, 2004
| A version of this story appeared in
Volume 82, Issue 37

A family of membrane-spanning proteins that mediate gas transport has finally yielded its secrets. Scientists at the University of California, San Francisco, have used X-ray crystallography to reveal the molecular architecture of a bacterial protein that ushers ammonia across cell membranes [Science, 305, 1587 (2004)]
This structure--the first of any gas channel protein--provides "a quantum leap forward in our understanding of gas transport," note biochemists Peter Agre of Johns Hopkins University School of Medicine and Mark A. Knepper of the National Institutes of Health in an accompanying Science commentary.
Ammonia is a gas, but in aqueous solution it exists predominantly as ammonium ion (NH4+). Bacteria use ammonia as a nitrogen source to make amino acids. In humans, ammonia is an important reactant in a variety of reactions. Although this crucial molecule can slowly diffuse through cell membranes on its own, nearly all organisms contain membrane-spanning channel proteins that allow ammonia to cross the lipid bilayer in a rapid and controlled manner.
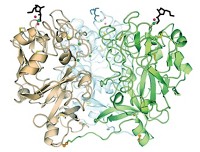
Professor Robert M. Stroud, postdoc Shahram Khademi, and their coworkers in the biochemistry and biophysics department at UC San Francisco have now solved the structure of a model bacterial ammonia channel to 1.35 Å, an unprecedented level of resolution for a membrane-spanning protein. The structure reveals that the channel is made up of three identical membrane-spanning subunits, each of which contains a 20-Å-long hydrophobic tunnel just wide enough for NH3 molecules to pass through single file. Wider vestibules flank each end of this narrow tunnel.

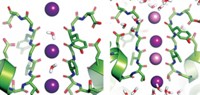
Stroud suggests that the constellation of aromatic residues in the extracellular vestibule binds ammonium ions from solution via  -cation interactions. In this vestibule, it appears that ammonium ions are converted to ammonia molecules. In the narrow tunnel beyond the vestibule, two histidine residues poking out of the tunnel's waxy wall stabilize the NH3 molecules via weak C–H···N hydrogen bonds. Because NH4+ ions can't accept any hydrogen bonds, they cannot pass through the tunnel, Stroud notes.
-cation interactions. In this vestibule, it appears that ammonium ions are converted to ammonia molecules. In the narrow tunnel beyond the vestibule, two histidine residues poking out of the tunnel's waxy wall stabilize the NH3 molecules via weak C–H···N hydrogen bonds. Because NH4+ ions can't accept any hydrogen bonds, they cannot pass through the tunnel, Stroud notes.

The channel's ability to discriminate against NH4+ is crucial because the protein must not alter the charge balance across the membrane by mistakenly letting K+ or other cations through. Indeed, Stroud's team has confirmed that the channel conducts NH3--and not ammonium ion--via a fluorescence assay using channel-studded vesicles loaded with a pH-sensitive fluorescent dye.
The mechanistic insights provided by the structure of this bacterial ammonia channel are likely to shed light on the workings of its human cousins, Stroud says. Those relatives include the Rh blood group proteins found on red blood cells--which are reported to ferry both ammonia and carbon dioxide across the cell membrane--as well as a number of physiologically important ammonia channels that regulate body pH and protect against ammonia toxicity. Stroud suggests that the structure might be used to understand the molecular basis of diseases caused by ammonia toxicity and perhaps even to design drugs to treat them.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter