Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Bug's Genetic Code Allows Pyrrolysine
The 22nd amino acid is biosynthesized directly, making possible expansion of genetic code
by Amanda Yarnell
October 11, 2004
| A version of this story appeared in
Volume 82, Issue 41

Recent work has shown that the 22nd genetically encoded amino acid, pyrrolysine, is biosynthesized as a free amino acid, something that hadn't been seen before outside of the "standard" 20 amino acids. This revelation has allowed an Ohio State University team to add pyrrolysine--thus far found only in an anaerobic microbe--to the genetically encoded amino acids that the model bacterium Escherichia coli uses to make proteins.
"Our study reveals that the natural world has used the same sort of strategy to expand the genetic code that chemists have used in the laboratory to artificially expand the genetic code for biotechnological applications," says Ohio State microbiologist Joseph A. Krzycki, who worked with chemist Michael K. Chan on the study. Chan's lab discovered pyrrolysine--an amide-linked 4-substituted pyrroline 5-carboxylate lysine derivative--in the crystal structure of a microbial methyltransferase enzyme [Science, 296, 1462 (2002)].
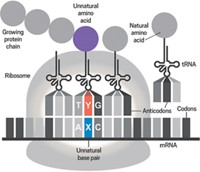
The 21 known genetically encoded amino acids are inserted into growing peptide chains by a dedicated transfer RNA (tRNA) that recognizes a specific three-nucleotide codon in the messenger RNA transcript. By isolating a pyrrolysine-specific tRNA, Krzycki's lab confirmed that pyrrolysine is genetically encoded, too [Science, 296, 1459 (2002)]. Earlier, his group had shown that a codon that's normally used to signal the end of a gene is located smack in the middle of the methyltransferase gene and that it directs the protein-making machinery to insert pyrrolysine instead of stopping. A similarly reassigned codon is used for selenocysteine, the 21st amino acid.
Previous work had suggested that pyrrolysine was not likely to be synthesized as a free amino acid, as the 20 standard amino acids are. Instead, Krzycki's lab figured pyrrolysine biosynthesis would parallel that of selenocysteine: A lysine would be attached to the pyrrolysine tRNA and then derivatized to give pyrrolysine. But recent results from the Ohio researchers, as well as independent work from Dieter Söll's lab at Yale University, have suggested otherwise.
To figure out how the new amino acid is incorporated into proteins, both the Ohio and Yale teams needed to make pyrrolysine, a task made more difficult by the fact that Chan's group initially could not tell whether the 4-substituent was a methyl, hydroxyl, or amino group. Söll's team elected to synthesize racemic pyrrolysine with a methyl substituent, having failed to make the hydroxyl version [Proc. Natl. Acad. Sci. USA, 101, 12450 (2004)]. Chan's team, however, used an elegant set of chemical modification, crystallography, and mass spectrometry experiments to prove that pyrrolysine's 4-substituent is a methyl group, then developed a way to chemically synthesize l-pyrrolysine [Chem. Biol., 11, 1317 (2004)].
With chemically synthesized pyrrolysine in hand, both the Söll and Krzycki labs set about testing whether the amino acid's tRNA prefers to bind lysine (as would be expected if pyrrolysine biosynthesis parallels selenocysteine biosynthesis) or pyrrolysine (as would be expected if pyrrolysine is biosynthesized in the same way the standard 20 amino acids are). To their surprise, both groups independently found that pyrrolysine (but not lysine) can be attached directly to the pyrrolysine tRNA by an enzyme they dub pyrrolysyl-tRNA synthetase. These results suggest that pyrrolysine is biosynthesized as a free amino acid and then attached to its tRNA. "This has never been seen before outside of the standard 20 amino acid set," Krzycki says.
THE UNEXPECTED result made Krzycki wonder whether his lab might be able to expand an organism's genetic code to accommodate pyrrolysine simply by introducing the genes for pyrrolysine tRNA and pyrrolysyl-tRNA synthetase and chemically synthesized pyrrolysine. As he had hoped, it worked. Bacteria containing the genes for the synthetase and the tRNA--as well as the methyltransferase enzyme that normally contains pyrrolysine--incorporate chemically synthesized pyrrolysine into the methyltransferase enzyme in the correct position with high efficiency [Nature, 431, 333 (2004)].
Krzycki points out that nature's strategy to include pyrrolysine in the genetic code parallels the one chemists have used to expand the genetic code to produce proteins containing novel amino acids: An artificial synthetase enzyme is created that attaches a novel amino acid to an artificial tRNA that inserts the novel amino acid at a reassigned stop codon.
Such artificial synthetase-tRNA pairs have proven valuable for creating proteins containing novel amino acids, but "no natural examples were known to exist," note Paul Schimmel and Kirk Beebe of Scripps Research Institute in an accompanying Nature commentary. "In some ways, this discovery reaffirms that notion that, if something can be done by experiment, it has probably been done earlier by nature," they note.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter