Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Making Peptides on Early Earth
Carbonyl sulfide may have mediated polymerization of amino acids
by Amanda Yarnell
October 11, 2004
| A version of this story appeared in
Volume 82, Issue 41
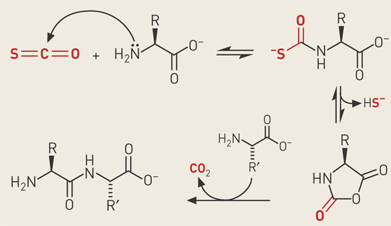
Whether formed on Earth or brought here by meteorites, α-amino acids are widely assumed to have been present in the prebiotic chemical soup. Recent experiments hint at how these amino acids might have been strung together to form peptides.
M. Reza Ghadiri and Luke L. Leman of Scripps Research Institute and Leslie E. Orgel of Salk Institute in San Diego demonstrate that carbonyl sulfide--a volcanic gas believed to be present in the atmosphere of prebiotic Earth--acts as a highly efficient condensing agent for the formation of peptides from amino acids in aqueous solution [Science, 306, 283 (2004)]. Depending on the reaction conditions and additives such as metal ions or oxidizing agents, peptide yields can go up to 80% in minutes to hours at room temperature.
These results suggest this simple, naturally occurring gas may have helped link amino acids into proteins on early Earth, Ghadiri says. "We believe that carbonyl sulfide is the most prebiotically relevant condensing agent yet described, with the possible exception of inorganic polyphosphates," he adds. Because carbonyl sulfide likely didn't last long in the early Earth's atmosphere, he suggests that the gas may have mediated polymerization of amino acids adsorbed to rocks in the vicinity of volcanic eruptions or near carbonyl sulfide-belching underwater vents.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter