Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Enhancing Natural Antidepressants
December 19, 2005
| A version of this story appeared in
Volume 83, Issue 51
Cannabis reputedly has an antidepressant effect due to the binding of its active constituent, Δ9-tetrahydrocannabinol, to cannabinoid receptors in the brain, according to Daniele Piomelli at the University of California, Irvine. The binding increases neuronal activity related to the control of mood. Cannabis isn't a feasible depression treatment, so Piomelli and colleagues are exploring an alternative: compounds that enhance the brain's naturally occurring cannabinoids, such as that shown. The researchers found that it "exerts potent antidepressant-like effects" in mice and rats (Proc. Natl. Acad. Sci. USA 2005, 102, 18620). The compound inhibits fatty-acid amide hydrolase, which hydrolyzes the endogenous cannabinoid anandamide. The inhibition leaves more intact anandamide to interact with cannabinoid receptors. The findings "point to fatty-acid amide hydrolase as a previously uncharacterized target for antidepressant drugs," the researchers note.
Dragging down metals with light
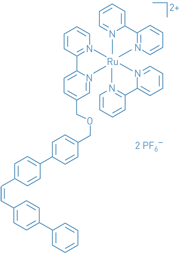
With their eyes on the haz-ards and economic implications of metal contaminants in the environment and in industrial reaction media such as those used to make pharmaceuticals, Craig S. Wilcox and Mark R. Ams of the University of Pittsburgh report what they assess as a "significant step" toward new types of metal-scavenging agents dubbed "precipitons" (J. Am. Chem. Soc., published online Dec. 13, dx.doi.org/10.1021/ja0561340). They have synthesized new precipiton structures that in solution undergo a swift bent-to-straight conformational change when illuminated with visible light captured by a light-absorbing appendage. That change can lead to precipitation of metal-containing complexes. They demonstrate the principle with a ruthenium-hubbed cluster of bipyridine molecules attached to a linear multiring ligand. Energy transfer from the ruthenium complex to the bent ligand causes it to straighten up. The next step is to expand this model system into a practical metal-scavenging approach.





Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter