Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Pharmaceuticals
Avian Influenza: A Hole In One
Cavity in N1 enzyme of H5N1 flu virus could be exploited for new flu drugs
by Michael Freemantle
August 21, 2006
| A version of this story appeared in
Volume 84, Issue 34
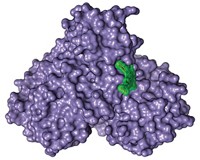
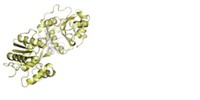
X-ray crystallography has revealed that the neuraminidase enzyme in the potentially pandemic H5N1 avian flu virus contains a cavity adjacent to its active site. That cavity presents new opportunities for the design of anti-influenza drugs, suggest John J. Skehel, director of the National Institute for Medical Research, in London, and coworkers (Nature, DOI: 10.1038/nature05114).
Neuraminidases facilitate the spread of influenza viruses during infection and are attractive targets for developing new drugs that work by inhibiting the activity of the enzymes. The nine subtypes of these enzymes fall into two genetically distinct groups: Group one contains N1, N4, N5, and N8; group two contains N2, N3, N6, N7, and N9.
Skehel's team determined the crystal structures of the N1, N4, and N8 subtypes and compared their active sites with those of the group two subtypes. They also determined the structures of the complexes formed when these subtypes bind to neuraminidase inhibitors such as Relenza and Tamiflu. These drugs were designed to block group two neuraminidases but they are also effective against N1-containing viruses such as H5N1. It was therefore assumed that the active sites of all nine subtypes are essentially the same.
The British researchers show that the conformation of a loop of amino acids, known as the 150-loop, is different for the group one and group two subtypes. In group one, the loop exposes a cavity adjacent to the active site that is not present in group two. When a neuraminidase inhibitor binds to the active site, the cavity collapses and the loop assumes a conformation similar to the group two loop conformation.
If one designed a drug that fit snugly into the cavity, it might be more effective against H5N1-like flu viruses than existing drugs, writes Ming Luo, professor of microbiology at the University of Alabama, Birmingham, in a commentary (Nature, DOI: 10.1038/nature05003).



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter