Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Reagent Nabs Enzyme Protein Substrates
November 27, 2006
| A version of this story appeared in
Volume 84, Issue 48
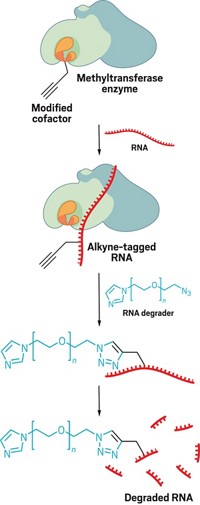
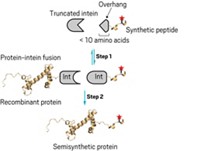
A reagent and a method used to identify the protein substrates of enzymes that install acetyl groups on proteins will make it easier for scientists to study how the acetyl chemical tags help orchestrate complex biological processes (J. Am. Chem. Soc. 2006, 128, 15356). Mounting evidence suggests that acetyltransferase enzymes, including those that modify the histone proteins that package human genetic material, add acetyl groups to far more protein substrates than once imagined, notes John S. Blanchard of Albert Einstein College of Medicine, Bronx, N.Y. His team's method can be used to reveal all substrates of a given acetyltransferase. The researchers start with a chlorinated version of the enzymes' normal acetyl group source, acetyl-coenzyme A. When this reagent and the acetyltransferase are added to cell extracts, the enzyme transfers a chloroacetyl group (green) to each of its protein substrates (red) to give the protein product shown. The chloroacetyl group serves as a reactive handle for substrate identification; addition of a cysteamine-derivatized rhodamine dye (blue) yields fluorescently labeled protein upon chloride elimination.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter