Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
The Secret Life Of Plant Crystals
Microscopic view of plant tissue reveals a hidden world of calcium oxalate crystals of diverse shapes
by Ivan Amato
February 6, 2006
| A version of this story appeared in
Volume 84, Issue 6

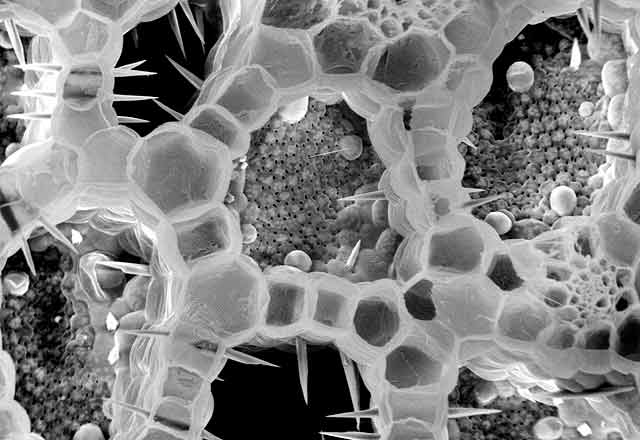
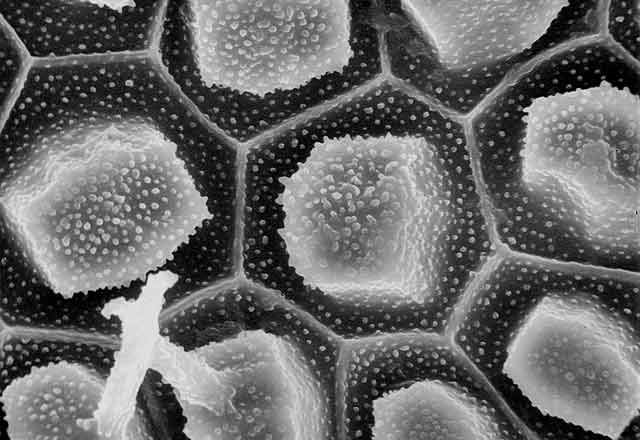

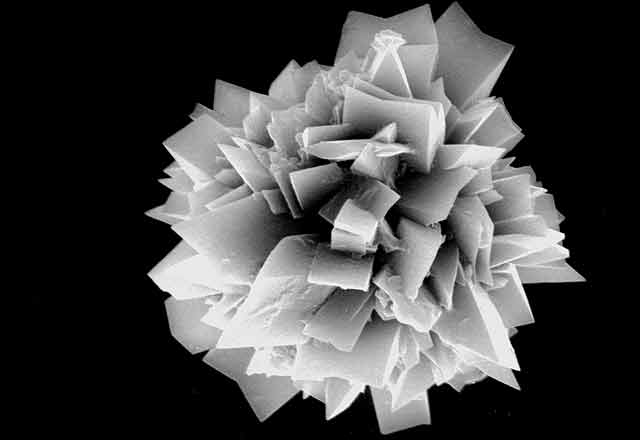
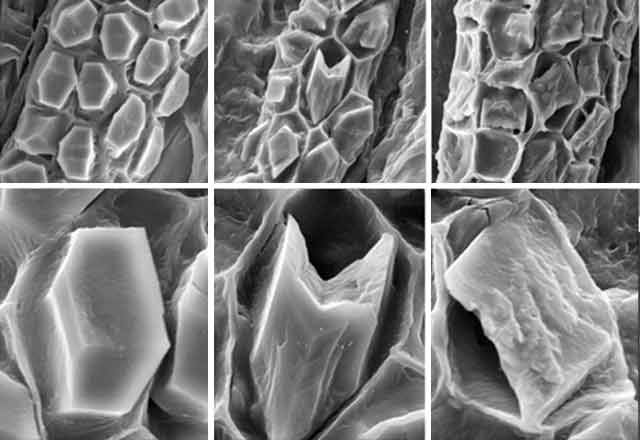
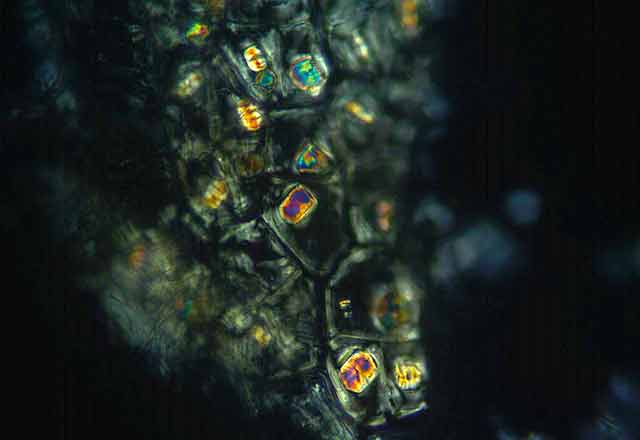
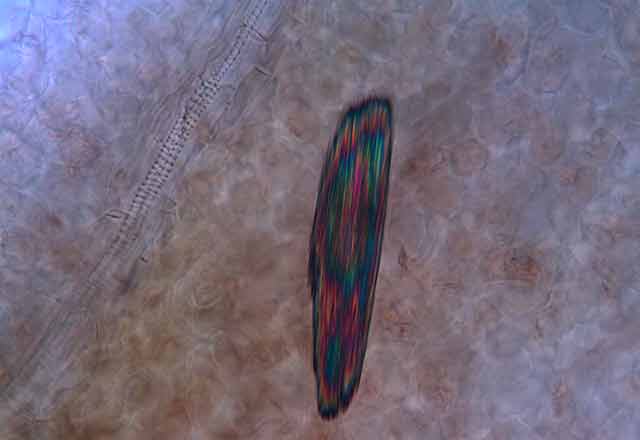
On Feb. 24, 2003, the manager of a cafeteria in Chicago contacted the city's Department of Health to report a spate of illnesses in people who had eaten at the facility three days earlier. Symptoms included burning and stinging in the mouth, difficulty in swallowing, and facial swelling. One person had enough trouble breathing to be admitted to an intensive care unit. For some, symptoms lasted for weeks.
In the ensuing investigation, health department researchers interviewed the cafeteria's staff and inspected the kitchen. Later, scientists at the Food & Drug Administration's Forensic Chemistry Center in Cincinnati conducted analyses of the "Chinese braised vegetable" entrée that all of the 10 affected people had eaten. Last year, in the journal Clinical Toxicology (2005, 1, 17), the team reported what they found: The entrée contained tiny javelin-shaped crystals known as raphides, and it was these crystals that most likely caused the troubling symptoms. "To our knowledge, this is the first reported foodborne disease outbreak associated with exposure to raphides," the researchers stated.
Raphides? Little known beyond a cadre of botanists, chemists, forensic investigators, and others interested in the interface of biology and crystals, raphides are one of several crystal forms of calcium oxalate found in plants. The biological functions of these crystals, which typically grow within individual cells, are still not completely understood.
"Seventy-five percent of flowering plants make one or more kinds of the crystals, and they form in specific places in tissues and organs," says Iowa State University's Harry T. (Jack) Horner, who has been studying calcium oxalate crystallization in plants for decades. Some plants make prismatic crystals, akin to miniaturized sugar grains. Others make "crystal sand" with less regular particle shapes that are shaped like little tetrahedrons. By far the most spectacular crystals are the ones known as druses. They look like microscopic carnations, but their rigid petals probably are sharp enough to lacerate the mouths of marauding insects.
That possibility strongly suggests to some researchers that one evolutionarily valuable trait of these crystals is to make it unpleasant, even downright dangerous, for a would-be herbivore to eat the plants. One plant known for its abundance of calcium oxalate crystals is dumbcane. Those who chew on it find that their mouth and tongue swell so much that it is painful and hard to talk.
Some raphides form within pressurized capsulelike cells that, when pierced or bitten into, forcibly expel the raphides and cell contents. In some plants, these raphides even have grooved faces that help to channel toxins made by the cell into tiny wounds created by the expelled crystals, notes molecular biologist Paul A. Nakata of the Department of Agriculture's Children's Nutrition Research Center, housed at Baylor College of Medicine in Houston. Last June in the Annual Review of Plant Biology, (2005, 56, 41), he and the late Vincent R. Franceschi of Washington State University provided an extensive status report on what is and is not known about calcium oxalate in plants.
Although the presence of calcium oxalate crystals was first described in plants by pioneer microscopist Antonie van Leeuwenhoek in the late 1600s, those few who study these crystals are quick to note that the structures' roles in plants, how plants manufacture the oxalate molecules that end up in the crystals, and how the crystals form remain open questions. "Most of us are interested in what factors control crystal cell development, and that gets down to the cellular, genetic, and molecular levels," Horner says.
Many researchers are convinced that calcium oxalate crystallization, which usually occurs within plant cells called crystal idioblasts, is the primary means for many plants to manhandle the calcium they often can't help but absorb from the soil into the low, nontoxic concentrations that their cells need. One line of evidence for this comes from studies in which crystal-forming plants grown in calcium-rich or calcium-poor environments produce more or fewer crystals, respectively.
Researchers have proposed, with varying degrees of experimental support, that calcium oxalate crystals, besides being involved in defense and calcium regulation, also sequester poisons including heavy metals; provide mechanical support; regulate ionic balances; and even, Horner says, help the plant out by gathering and focusing light onto photosynthetic pigments enclosed in chloroplasts.
Compared with tooth, bone, and shell formation, calcium oxalate crystallization is an unsung example of biomineralization. Yet it plays roles in everyday plant life and survival and in human health. Calcium oxalate, after all, is one of the compounds that accrete into kidney stones in people.
In the hands of Élan Sudberg, chief operating officer of a contract analysis firm called Alkemists Pharmaceuticals in Costa Mesa, Calif., calcium oxalate crystals even serve for making taxonomic and forensic identifications, such as of adulterants in dietary supplements. "The combination of the particular crystal types, their location in the plant, as well as other cellular characteristics are meaningful in identifying the genus and species of many botanicals," Sudberg says.
Displayed on these pages is a gallery of optical and electron microscope images that reveal the diverse ways in which plants sculpt calcium oxalate crystals into functional forms that are visually stunning and, at times, physically stunning to those they pierce, whether they be caterpillars or cafeteria diners.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter