Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Pharmaceuticals
Living On The Edge
Drugs targeting the protein Hsp90 push already unstable cancer cells to the brink
by Lisa M. Jarvis
February 26, 2007
| A version of this story appeared in
Volume 85, Issue 9
WHEN LAURENCE PEARL, professor of protein crystallography at the Institute of Cancer Research, in London, used to visit pharmaceutical companies to discuss the potential of heat shock protein 90 (Hsp90) as an anticancer target, "they listened politely, gave me a cup of coffee, and showed me the door," he recalls. His hosts suggested that Hsp90 was of "great academic esoteric interest" but would never be taken seriously as a drug target. "These days, they have discovered their mistake," Pearl says.

These days, Hsp90, a molecular chaperone, has come into its own as a tantalizing target for cancer therapies. University researchers have made major strides in explaining how Hsp90 helps disease survive, while investigators at biotechnology companies have developed an assortment of small molecules that block the protein. Those prospective drugs finally are making their way through to market, piquing the interest of big pharmaceutical companies hungry for new products.
Several years back, the naysayers may have had a point. As recently as the early 1990s, scientists knew little about Hsp90's complex role in cancer cells. The one undisputable fact was that the protein was critical to the survival of both normal and sick cells. It was thus reasonable to doubt the wisdom of wiping out something that functioned on such a basic level.
"There was a lot of resistance to going after something that is considered a housekeeping protein," says Leonard M. Neckers, a senior investigator in the urology oncology branch of the National Cancer Institute (NCI), Frederick, Md., whose pioneering research in the late 1980s and early 1990s helped put Hsp90 on the map. The concern was that any drug that knocked out the protein would be lethal because healthy cells would be just as vulnerable as sick ones.
But over the years, researchers in academia as well as industry have paved the way for drug development by helping to define Hsp90's complicated role in protein maintenance.
DESPITE THEIR rather dramatic-sounding name, heat shock proteins were long considered to be no more than the cell's housekeeping crew. Under normal conditions, their activities are fairly mundane; they help newly made proteins fold properly, cart off old proteins to the cell's recycling center, and generally spend much of their time waiting around for something to happen.
But when the cell is under stress, either from genetic mutation or from environmental changes such as infection or heat—hence the proteins' name—these proteins transform into an emergency response team. Their job is to run triage for the cell, stabilizing partially unfolded proteins, helping some to twist and turn into the right shape, and keeping the wrong proteins from building up.
As basic as these tasks sound, they are hugely important. In a human cell, proteins unable to maintain their proper shape eventually are broken down and swept out. That's all well and good if they are mutated and dangerous for the cell's survival, but it's problematic if the proteins are needed for normal functioning.
Yet Hsp90 is a special kind of heat shock protein; although others in its class are usually promiscuous in their interactions—any protein out of the thousands that come along will do—Hsp90 is far more selective about which proteins it will help. "Proteomics analysis shows that Hsp90 is probably physically interacting with about 300 proteins," notes Beatrice Darimont, an assistant professor at the University of Oregon. Darimont recently reported research findings that pinpoint the docking site for one of those proteins, the glucocorticoid receptor.
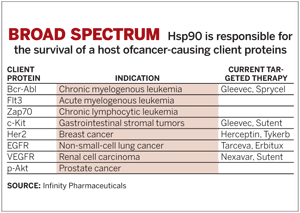
More important, the majority of the 300 proteins that Hsp90 interacts with—known as client proteins—have regulatory functions. Many of these regulatory proteins play a role in pathways that are impacted by cancer.
And in a coup for drug companies, it appears that drugs that inhibit Hsp90 are somehow more selective for the Hsp90 in cancer cells than for that in normal cells. Exactly why is under debate; tumor cells are certainly on the edge of survival and thus should be more reliant on Hsp90, but that does not explain why the small molecules are more selective for Hsp90 in tumors, Neckers notes. Regardless, that selectivity allows a cancer patient to be given an Hsp90 inhibitor without any real concern about knocking out important functions in healthy cells.
From there, the Hsp90 story becomes more complex. Not only is Hsp90 selective regarding the proteins it interacts with, but there is an entire machinery of other heat shock proteins and chaperone proteins that form complexes with Hsp90 to assist in introducing it to its clients.
Scientists are just beginning to understand the carefully choreographed dance between Hsp90, its chaperones, and its client proteins. Because these client proteins are diverse in their structure and function, scientists have been baffled by how Hsp90 is able to distinguish among them.
"The good news is that this year we got the information we were waiting for," Darimont says. Last April, Pearl and his colleagues at the Institute of Cancer Research published the complete crystal structure of Hsp90 bound to the energy-transfer nucleotide adenosine 5′-triphosphate (ATP) (Nature 2006, 440, 1013). They showed how Hsp90 hydrolyzed ATP, a major breakthrough since Hsp90 must hook up with ATP to accomplish its job of helping other proteins be the best they can be.

"The binding and metabolism of ATP is absolutely critical for Hsp90's chaperoning function," points out Andrew Massey, principal scientist at the U.K.-based biotechnology firm Vernalis.
Importantly, this new information about Hsp90's mechanism could be useful for companies looking to develop drugs that inhibit the protein, since all of the current Hsp90 inhibitors target the very point where Hsp90 and ATP join.
"One of the pleasing things about Hsp90 as a drug target from a chemist's point of view is that the pocket is a very deep, very defined binding site with very few direct contacts between ATP and the protein itself," Pearl says. The space is flexible enough to accommodate drugs with a range of structures.
Researchers also discovered that the regulatory proteins associated with cancer seem to have specific docking sites on Hsp90. This could open the door for drugs that are more attuned to certain pathways, Darimont says. "If you can manipulate specific interactions, then you can actually manipulate specific pathways without others that are dependent on Hsp90 being affected."
While academia fills in some of the blanks in the science behind Hsp90, interest from industry has started to heat up. In the past year, Biogen Idec has acquired Conforma Therapeutics, MedImmune has licensed Infinity Pharmaceuticals' Hsp90 program, and Novartis has deepened its commitment to Vernalis' Hsp90 program by choosing a second drug candidate to put into the clinic. Industry observers say almost every big drug company is looking at this opportunity, and several small biotech firms are pursuing it as well.
"It is becoming an increasingly competitive landscape," says Martin Drysdale, director of chemistry and structural science at Vernalis. "There are certainly a lot of people interested in Hsp90, and more companies are coming out of the woodwork."
The new clarity in the science behind the protein's activity accounts for some of the heightened interest, but a growing trend toward broader approaches to disease treatment is also drawing companies to the class. "I think the mood for big pharma has changed over the past five years or so from focusing on highly specific, molecularly targeted agents" to concentrate on drugs that have a wider range of activity, NCI's Neckers notes.
Companies recognize that while targeted drugs like Novartis' leukemia treatment Gleevec set up a roadblock squarely in a disease's path, making such drugs highly effective for a period of time, cancer cells eventually will find a side street. In the case of Gleevec, several next-generation drugs that tackle resistance are already in development.
As a result, companies are always one step behind the cancer, notes Marcus Boehm, Biogen Idec's senior director of chemistry. "It's a catch-up game, where you're always trying to make another molecule to overturn the resistance," he says.
THE THEORY behind targeting Hsp90, in contrast, is that drug-resistant proteins are in their most unnatural form, which is when they are most in need of help from Hsp90 to fold and stay alive. "You're really going to the end-stage of the game, saying 'give me the most mutated protein you can get, and that's the one that will be the most vulnerable to inhibition of Hsp90,'" Boehm says.
"We're not necessarily in the business of killing cancer cells; we're in the business of inducing cancer cells to kill themselves. It's a much more civilized way," he adds.
In short, if Hsp90 inhibitors live up to their potential, they could circumvent that cycle of research devoted to creating "the grandsons and great-grandsons" of drugs like Gleevec, explains Jeffrey Tong, vice president of corporate and product development at Infinity.
While macro trends in oncology drug development are clearly contributing to the interest in Hsp90, funding from big pharma and other outside investors rarely materializes until a drug class shows promise in the clinic. And Hsp90 inhibitors are finally starting to generate data. Patients being treated with maximum doses of Hsp90 inhibitors are getting a strong enough response to tweak the industry's excitement, says David Parkinson, Biogen Idec's senior vice president of oncology R&D.
"We're beginning to get a sense of what a clinical development strategy for these agents should look like-the kinds of diseases, doses, endpoints, and the types of drugs that would really make a difference," he adds.
Companies in the Hsp90 field fall into two main camps: those pursuing drugs based on the natural products that were first identified as inhibiting the protein and those pushing small molecules designed around the structure and function of Hsp90 bound to ATP and its client proteins. Both camps are attacking the same binding site, the pocket created when Hsp90 hooks up with ATP.
The natural product camp is focused mainly on geldanamycin, an antibiotic in soil microorganisms that was the first molecule found to inhibit Hsp90. Geldanamycin was discovered back in the 1980s. The journey to viable drugs that inhibit Hsp90 has been so lengthy because the antibiotic's structure is fundamentally flawed from a medicinal chemist's perspective.
"The development of geldanamycin at NCI was excruciatingly slow for all sorts of technical reasons," notes Parkinson, who worked on geldanamycin for many years at NCI before joining Biogen Idec.
It turned out that geldanamycin, although extremely potent, has a quinone moiety that turns it into an electrophile. In addition to being highly reactive, the compound is also highly insoluble-a deadly combination in drug discovery.
MUCH OF THE early research that led to the most advanced drugs in the clinic was devoted to overcoming that pesky quinone, either by designing around it or removing it all together.
Kosan Biosciences has, by all accounts, paved the way for the current smattering of Hsp90 inhibitors in the clinic. The company's efforts, which are focused on geldanamycin derivatives, helped to eradicate much of the uncertainty around Hsp90 inhibitors, claims Robert G. Johnson, Kosan's president and chief executive officer.
Most important, Kosan was the first to offer solid early-phase clinical data validating the therapeutic rationale behind Hsp90 inhibitors. "We have patients taking our first-generation agent who have been on the drug for well over a year," Johnson says, and a second-generation product is almost as far along. "We're seeing areas of efficacy in terms of tumor reduction in both," he notes.
Kosan's most advanced compound, tanespimycin, is a proprietary formulation of 17-AAG (17-allylamino-17-demethoxy-geldanamycin), the first analog of geldanamycin that the company has investigated. The drug retains the quinone found in geldanamycin but replaces a methoxy arm with an allylamino group to minimize the reactivity problem. Although that tweak does not address geldanamycin's lack of solubility, Kosan devised a formulation that overcomes that limitation as well.
Kosan unveiled promising Phase II clinical trial data for tanespimycin in Her2-positive breast cancer in December. The drug produced significant tumor shrinkage in patients whose tumors had progressed even after taking Genentech's monoclonal antibody Herceptin.
Advertisement
In addition to the breast cancer trial, Kosan is pursuing Phase II trials for tanespimycin in patients with multiple myeloma and melanoma. Later this year, the company intends to initiate Phase III trials to test tanespimycin in combination with Millennium Pharmaceutical's drug Velcade for those suffering from multiple myeloma.
Though tanespimycin showed promise in the breast cancer trial, Kosan is switching gears to focus its efforts in that indication on its second-generation compound, alvespimycin. The drug is a formulation of 17-DMAG (17-dimethylaminoethylamino-17- demethoxygeldanamycin), an analog with side-chain modifications that, according to Kosan, improve the drug's half-life and potency. The drug is water soluble, which means it can be administered intravenously or orally.
Kosan, which has yet to partner either of its Hsp90 inhibitors with other drugs, plans to move an intravenous form of alvespimycin into Phase II monotherapy studies in Her2-positive breast cancer this year, Johnson says, followed by Phase II/III combination therapy studies. An oral formulation of the drug will soon enter clinical trials for cancer types, such as hematological tumors, where taking a pill is standard.
If 17-AAG and 17-DMAG are the first and second cousins of geldanamycin, then Infinity's lead compound is several generations removed from its forebearer. While early attempts to overcome the reactivity and solubility hurdles relied on replacing appendages on geldanamycin's quinone component, Infinity researchers removed the problematic quinone altogether by reducing it to a hydroquinone.
"That changes the electronic properties of the nitrogen, and all of a sudden, we could make the hydrochloride salt," Infinity's Tong says. The resulting compound, IPI-504, is 4,000 times more soluble than 17-AAG, enabling both an intravenous and an oral formulation, without the reactivity of geldanamycin.
Tong is encouraged by early results from a Phase I trial of an intravenous IPI-504 formulation in patients with gastrointestinal stromal tumors (GIST) that have grown resistant to Gleevec. Although Phase I trials are designed to determine the safety of a drug and establish a dosing schedule, researchers saw signs that the drug could induce a response in tumors. PET scans, used to track a drug's activity in patients with GIST, suggested that tumors responded when patients were given IPI-504, only to proliferate when the patient was taken off the drug and abate again when the patient was put back on.
On the basis of these results, Infinity and partner MedImmune altered the trial to eliminate the drug holiday. The companies plan to substantially expand the clinical program for IPI-504 this year. The goal is to complete the GIST trial, initiate a Phase I/II trial in non-small-cell lung cancer, and launch a clinical program for the oral version of IPI-504.
Though Kosan and Infinity remain confident that the problems associated with geldanamycin can be overcome, others, even those who have spent a lot of time and energy on derivatives of the natural product, are not convinced it is the best route to blocking Hsp90.
Biogen Idec entered the Hsp90 market last spring when it acquired Conforma Therapeutics, a San Diego-based firm founded in 1999 to develop drugs targeting Hsp90. Like Kosan's, Biogen Idec's most advanced molecule is a unique formulation of 17-AAG. However, Biogen Idec has opted to put that molecule on the back burner. "We just don't think it'll be competitive with what we can do with our other small molecules," Parkinson says.
"Initially, we made hundreds of geldanamycin derivatives," explains Boehm, former vice president of chemistry at Conforma. "We found that overall they are very similar to each other and that there are limitations to what you can do with them."
One problem, Boehm says, is that geldanamycin triggers the action of p-glycoprotein (Pgp), an ATP-dependent pump that flushes drugs from cells before they can do their work. This resistance mechanism can be dampened or prevented by administering other drugs along with the Hsp90 inhibitors, but Biogen Idec believes a better route is to avoid the issue altogether.
Biogen Idec is now pushing into the clinic a next generation of Hsp90 inhibitors that are "entirely designed, synthetic molecules with no relationship to geldanamycin," aside from the propensity to latch onto the ATP-binding site of the protein, Boehm says. The company based the design of its molecules on ATP.
These next-generation drugs also sidestep the Pgp mechanism. As a result, the compounds can better enter certain tissues and potentially can combine more easily with other cancer drugs, Boehm says. Conforma now is preparing an Investigational New Drug Application for the next-generation drugs and is "aggressively moving forward with a whole series of planned trials," Parkinson adds.
Vernalis is also taking a novel approach to the Hsp90 problem, tapping into its expertise in structure-based drug discovery to design molecules that overcome some of the earlier challenges, says Drysdale, the firm's chemistry director.
The geldanamycin compounds were "real groundbreakers" in opening up a market for Hsp90 inhibitors, Drysdale says, but Vernalis avoided the approach out of concern for potential liabilities. Despite side-chain modifications and the like, "they're all still 17-AAG by any other name," he says. "If there are going to be issues with that class, then all of these 17-AAG flavors are going to have the same issue."
Drysdale believes the next generation of specially designed molecules will have a broader spectrum of use. For its part, Vernalis deployed fragment-based drug discovery technology to find low-molecular-weight compounds with a weak binding affinity for Hsp90. Guided by X-ray crystal structures of prospective compounds bound to Hsp90, company researchers optimized the compounds into leads. The end result is a range of drugs that do not all fall under the geldanamycin umbrella.
Novartis licensed Vernalis' Hsp90 program in 2004 and has since funded the company's research in the area in exchange for worldwide marketing rights to its small-molecule Hsp90 inhibitors. Two compounds out of the collaboration have progressed into the preclinical stage, and one of those is expected to start Phase I trials in mid-2007. Novartis will handle clinical development.
Durham, N.C.-based Serenex says its Hsp90 program is based on a chemical scaffold distinct from even the newer generations of Hsp90 inhibitors. Using its screening technology, which had been used years earlier to identify some of the first geldanamycin analogs, the company tested thousands of compounds against ATP and other targets with purine binding sites.
Serenex didn't start out pursuing Hsp90 but rather generated hits for well over 100 protein targets. It then looked for targets from that collection "where our technology could give us an advantage such that a small company—our research group is fewer than 20 people—could compete," says Steven Hall, Serenex' senior vice president of R&D.
"Hsp90 rose to the top of that list," Hall says. "It was a targeted therapy, going after a specific biochemical target, but by inhibiting that one protein, we could block multiple signaling pathways."
The company says its lead drug, SNX-5422, is a synthetic, water-soluble compound with a wider therapeutic window than some of its geldanamycin-based predecessors. The company has already had an early discussion with the Food & Drug Administration and is hoping to win approval to put SNX-5422 into Phase I trials by early in the second quarter.
AT LEAST ONE newcomer, Cambridge, England-based Biotica, is sticking to the backbone of the class of antibiotics into which geldanamycin falls. But the company believes its technology can impose enough of a difference in the molecular structure to overcome the problems inherent in the natural product, says Ming-Qiang Zhang, the firm's vice president of research.
Biotica says its technology lets it file through each step of the biosynthesis of the natural product and determine which gene encodes the enzyme responsible for catalyzing each step. Biotica then reverse-engineers that multistep process such that specific genes are deleted one by one or altered to give the pathway a new set of instructions. The result is a new molecule—ideally, without the kinks that limited the natural product—that can be cheaply produced through biosynthesis.
That technology was deployed to alter the structure of macbecin, a natural product that, like geldanamycin, is part of the ansamycin class of antibiotics. Although still in the early stages of development, the drug is showing promise, Zhang says. In vivo and in vitro safety and efficacy data lead the company to believe its compound has a significantly broader therapeutic window than the geldanamycin derivatives.
Even though it is early days for all the drugs in the pipeline, companies already are plugging away at a follow-on generation of Hsp90 inhibitors. These compounds are informed by the more complete picture coming out of academia of the structure and mechanism of complexes of ATP, Hsp90, chaperone proteins, and client proteins.
For example, Serenex' efforts to develop novel versions of Hsp90 inhibitors are guided in part by recent strides in obtaining structural information about how Hsp90 interacts with its client proteins. The first generation of Hsp90 inhibitors is, in theory, blocking all the functions of Hsp90 equally, Hall explains. Serenex is now attempting to design molecules to block specific aspects of Hsp90 activity, with the hope of extending efficacy and the therapeutic window.
Advertisement
And even though researchers have significantly narrowed the knowledge gap surrounding Hsp90's mechanism of action, there are still major gaps that need to be filled in before the protein can be fully harnessed. "We're still almost completely ignorant as to what Hsp90 is doing to client proteins," says Pearl of the Institute of Cancer Research.
There are clues. The conformational steps, the identity of some of the regulatory proteins, and the binding of those proteins and their biomechanical function are all known, but "how all of that conspires to activate a client protein is unknown," he notes.
Hoping to fill out that picture, Pearl's team currently is attempting to replicate in the lab a complex of Hsp90, a client protein, and one of the chaperones. "We need a picture that has all the proteins intact," Biogen Idec's Boehm agrees. "That's a huge challenge."
A related question is why Hsp90 appears to be more active in cancer cells than in regular cells. "It looks like not all Hsp90 is equivalent in terms of its sensitivity to these inhibitor drugs," NCI's Neckers says. Researchers speculate that there is a subfraction of Hsp90 with a high affinity for the small-molecule inhibitors and that the subfraction may be more abundant in tumor cells than in normal cells.
Neckers' team is trying to identify whether there is a molecular signature for that supersensitive subfraction of Hsp90-that is, some alteration that occurs after the protein is synthesized that is vital for its interaction with inhibiting drugs. Current evidence points to an acetylation site on certain Hsp90 proteins that appears to distinguish them from the larger Hsp90 pool.
"I think in the next five years or so, there is going to be a lot more known about the various changes that Hsp90 can undergo and how these changes affect function," Neckers says.
Researchers say they are up for the challenge. As Pearl says, "We will spend the next 10 years learning how this machine works."
MORE ON THIS STORY



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter