Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Enzyme Opens For Business
Transglutaminase 2 structure may pave new route to developing treatments for celiac disease
by Stu Borman
January 7, 2008
| A version of this story appeared in
Volume 86, Issue 1
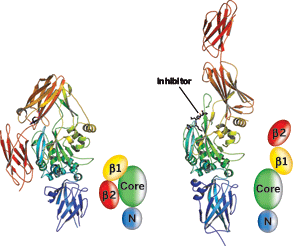
TG2 has previously been seen only in its "closed" conformation (left). When TG2 binds an inhibitor, the enzyme converts to an "open" conformation. Models depict TG2's β1 and β2, catalytic core, and N-terminal domains for each structure.
CELIAC DISEASE, a condition that damages the small intestine and prevents nutrient absorption, is an autoimmune reaction both to an external substance—the protein gluten in wheat, rye, and barley—and an internal enzyme called transglutaminase 2 (TG2). But the way gluten and TG2 act together to cause celiac disease is largely unknown. A new structure of the enzyme sheds some light on that interaction and may even point the way to developing treatments for the disease.
TG2 adopts an "open" conformation when it binds gluten, and it is this form of the enzyme that is believed to initiate an autoimmune reaction in susceptible individuals. Several groups have been trying for some time to obtain the structure of TG2 in this open conformation. The structure has proven elusive, however, because TG2 aggregates easily and is extraordinarily hard to crystallize.
Now, a research team has managed to determine the structure of the open form of human TG2 for the first time. Chaitan Khosla, professor of chemistry and chemical engineering at Stanford University, and coworkers obtained the 2-?? X-ray crystal structure (PLoS Biol. 2007, 5, e327).
The researchers succeeded where others haven't by trapping the open state of TG2 with an inhibitor structurally similar to key gluten peptides. The achievement could aid additional studies of TG2 and could help researchers design inhibitors of the enzyme that could serve as medications for celiac disease and other TG2-related conditions.
The X-ray crystal structure of human TG2 has been solved before, but only with the enzyme in a "closed," inactive form in which its active site is inaccessible to substrates. A 2002 TG2 structure by structural biologist Jon Clardy of Harvard Medical School and coworkers "revealed a lot about TG2, but it also made clear that there had to be large-scale rearrangements for catalysis," Clardy says. The new structure "reveals what these rearrangements are," clarifies the enzyme's mechanism of action, "and provides key insights for drug design," he explains.
The open structure of TG2 obtained by Khosla and coworkers is radically different from that of the closed conformation. The enzyme has four domains, and when it converts from its closed to its open form, two of the domains swing about a hinge region of the structure and move to a completely different location in the protein. This process also appears to expose the active site, opening it for business.
"The surprising aspect of the structure is the large degree of conformational change the enzyme undergoes," says chemistry professor Jeffrey W. Keillor of the University of Montreal, whose group has studied the mechanism and inhibition of TG2. "It completely unfolds from a compact form to a wide-open, linear form."
Some of the protein's residues move as much as 120° in the conformational change. Large changes of this type have been observed before in large, multidomain proteins, but they are rare.
"The findings will have widespread impact in the field because there have been so few structures of TG2, and none with a ligand bound in the active site," Keillor says. His group has been trying to determine such a structure as well, but to no avail.
CRYSTALLOGRAPHER Bijan Ahvazi and coworkers at the National Institutes of Health have been trying to do the same thing for six years and have recently had some success. "It's a wonderful structure," he says of the work by Khosla and coworkers. "It matches what we have, but we haven't published yet."
Khosla and coworkers believe the celiac autoimmune reaction may be sparked in susceptible individuals by the open conformation adopted by TG2 when it binds dietary gluten peptides. "While the relationship between the X-ray structure of open TG2 and celiac pathogenesis is hypothetical at present, it is intriguing," Khosla says.
"Certainly, it's an important structure," says professor Laszlo Lorand of Northwestern University's Feinberg Medical School, who has been studying mammalian transglutaminases for more than 40 years. "It gives you a better chance of dreaming up new inhibitors" that could be useful therapeutically, he adds.
Chemistry professor Richard A. Cerione of Cornell University, a coworker of Clardy's on the 2002 TG2 structure, says the new one "is something people in this field have been waiting for. Our group has been examining the role of TG2 in cancer cell growth, migration, and invasive activity. So knowing how a substrate or analog sits in the active site has potentially useful biomedical implications that probably go well beyond celiac disease."


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter