Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Antibiotic Ideas
Studies advocate blocking cell-division protein, essential metabolic pathway
by Carmen Drahl
September 22, 2008
| A version of this story appeared in
Volume 86, Issue 38

WILY BACTERIAL STRAINS are increasingly outwitting antibiotics, but only two new classes of the drugs have been introduced in the last 40 years. Now, two new studies point to additional targets for antibiotic weaponry.
In one study, an international team of researchers coordinated by David J. Haydon of Prolysis, a company specializing in antibacterials, has shown that blocking the protein FtsZ, a bacterial relative of the cell-division protein β-tubulin, is a viable antibiotic strategy (Science 2008, 321, 1673). Meanwhile, a team led by Tohru Dairi of the Biotechnology Research Center at Toyama Prefectural University, in Japan, has uncovered another potential target—an alternative biosynthetic pathway for menaquinones, which are essential electron-transfer compounds for many pathogens (Science 2008, 321, 1670).
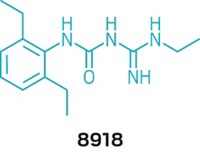
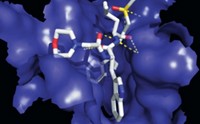
Many groups are studying FtsZ as a potential drug target, but the Haydon team's work is "a major advance," says Shahriar Mobashery, a University of Notre Dame chemist who specializes in antibiotics. Until this work, researchers hadn't convincingly shown that blocking FtsZ could kill bacteria, explains Lloyd G. Czaplewski, Haydon's Prolysis colleague and coauthor. Using the technique of fragment-based drug design, the team developed an initial screening hit into antibiotic PC190723. The compound saved mice from lethal doses of Staphylococcus aureus and killed multidrug-resistant S. aureus in culture. Molecular models suggest that PC190723 binds in a pocket adjacent to FtsZ's active site, and additional binding studies indicate that it doesn't interact with human tubulin, Czaplewski says. The scientists declined to disclose their plans for the compound.
Dairi's team screened genome databases and found that some bacteria use a previously unknown series of enzymes to produce menaquinones, electron-transfer compounds that vary in the length of their aliphatic side chains. They further showed that the new pathway is critical for pathogens that cause illnesses such as syphilis but not for humans or beneficial bacteria in the human gut. This makes the pathway an attractive option for making targeted antibacterials, Dairi says.
Bacterial genome sequencing efforts have been very important for the research community, but they alone don't tell the whole story, Mobashery says. Dairi's work is "a tour de force," he adds, because of how the team painstakingly decoded genomic data to discover a functioning metabolic pathway. "We hope to find other unique metabolic pathways in bacteria with the same methods," Dairi notes.
These reports follow a recent study that offers additional support for another antibacterial strategy: keeping bacteria from becoming infectious rather than killing them (Science 2008, 321, 1078).





Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter