Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
The First Four
The founding generation of epigenetics-based drugs combine promise and peril
by Ivan Amato
April 6, 2009
| A version of this story appeared in
Volume 87, Issue 14

Last August, the Food & Drug Administration gave the green light for doctors to prescribe Vidaza to patients with dire forms of myelodysplastic syndromes, a set of bone marrow conditions that often progress into terminal blood cancers. With this treatment, some of the 20,000 mostly older people who are newly diagnosed with MDS each year may be spared the quality-of-life blows that come with aggressive treatments such as weekly blood transfusions.
"Most MDS patients' lives circle around transfusions," says Kathy Heptinstall, operating director of the MDS Foundation. "With Vidaza, many patients become transfusion independent." And compared with the conventional care regimens for MDS, Vidaza can give a patient a few extra months, maybe an extra year, to live. "This is the first time there is demonstrable proof that a therapy can lengthen life, especially for high-risk MDS patients," Heptinstall says.
Far more consequential than the measured therapeutic benefits Vidaza has brought to some desperately ill patients is the drug's mode of action: An analog of the RNA-building block cytidine and known as 5-azacitidine, the drug works—at least in part—by fiddling with the switching mechanisms on chromosomes that turn genes on and off. In Vidaza's case, this intervention occurs by way of removing gene-silencing methyl groups along overly methylated stretches of DNA. Most effective in proliferating cells such as cancer cells, Vidaza, sold by the Summit, N.J.-based biopharmaceutical company Celgene, is one of only four so-called epigenetics-based drugs available in the clinic.
According to FDA's clinical trials database, upward of 200 clinical trials with both existing and new epigenetics-based drugs are in various stages of planning and completion. And medicinal chemists have been churning out new candidates by the hundreds. A recent review article by researchers from the Italian pharmaceutical firm Menarini Ricerche catalogued hundreds of so-called HDAC inhibitors (J. Med. Chem. 2008, 51, 1505). Members of this class of epigenetics-based medical agents work by blocking the removal of acetyl groups from specific lysine residues in some of the histone proteins closely associated with DNA in chromosomes. In the past five years, the authors note, more than 100 patents claiming new chemical series of HDAC inhibitors have been published. The market research firm Fuji-Keizai USA calculates that the 2009 global value for epigenetics-based research tools, diagnostic kits, and drugs will amount to $1.7 billion.
There is a perilous side to leveraging epigenetics mechanisms in pursuit of therapeutic payoff. The wrong kind of epigenetic interventions can silence a gene or turn it on when it shouldn't be. Either way, the biological responses can be indistinguishable from those due to deleterious genetic mutations.
This is what makes Vidaza, vorinostat (suberoylanilide hydroxamic acid, or SAHA, an HDAC inhibitor sold by Merck & Co. as Zolinza), and the two other clinically approved epigenetics drugs (valproic acid, a long-prescribed anticonvulsant that is also an HDAC inhibitor, and decitabine, a demethylating agent like Vidaza) so harrowing to drug developers. They all work in a genomically global fashion, not merely on particular genetic stretches that might be implicated in a disorder. This first generation of epigenetics-based drugs, therefore, alters the activity of many genes, for better or for worse. From the metric of drug specificity, "these are awful compounds," says epigenetic researcher Norbert O. Reich of the University of California, Santa Barbara. They only work at all, because, like conventional chemotherapies, they can do more harm to a patient's disease-associated cells than to the healthy cells, he notes.
This worries Melanie Ehrlich, a molecular biologist at Tulane University, in New Orleans, and founder of the DNA Methylation Society. Analyses of methylation patterns have revealed that cancerous cells and tissues often are associated with genome-wide reductions of DNA methylation. "Moreover, tumor progression is frequently marked by these decreases in DNA methylation," she says. "So a sledge-hammer attempt to decrease DNA methylation in cancer patients may lead to more metastases later on." A demethylating agent that saves you today could end up killing you later, she says.
But Ehrlich, Reich, and others also point out that Vidaza, SAHA, valproic acid, and decitabine constitute the first generation of what is likely to become a major therapeutic strategy. In time, they predict, pharmaceutical researchers will develop more sophisticated agents that target genomic locations, perhaps using recognition-components such as antibodies or snippets of RNA. Moshe Szyf of McGill University, where he is working to tease out environmental conditions that can have epigenetic consequences, says, "The potential for epigenetics medicine is just enormous, from both the diagnostic and therapeutic standpoints."

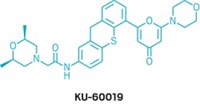
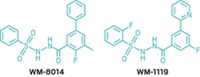
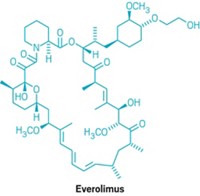

Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter