Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Alzheimer's Scary Link To Diabetes
Disruptions of insulin signaling and glucose regulation contribute to development of Alzheimer's disease
by Sophie L. Rovner
May 18, 2009
| A version of this story appeared in
Volume 87, Issue 20
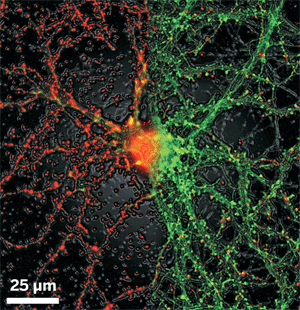
AS IF BLINDNESS, heart disease, nerve damage, kidney failure, and other serious complications were not enough for people with diabetes to worry about, now biomedical researchers are recognizing another apparent complication: Alzheimer's disease.
Diabetics tend to get Alzheimer's more frequently, and possibly at an earlier age, than the general population, according to David R. Schubert, a neurobiologist at the Salk Institute for Biological Studies, in La Jolla, Calif. People with type 2 diabetes face a 50–100% higher risk of developing Alzheimer's than nondiabetics, he says. Some studies show that just having high blood sugar may be sufficient to cause cognitive problems.
The implications of an association between Alzheimer's and diabetes are chilling. About 5 million Americans already have Alzheimer's disease, according to the Alzheimer's Association. Approximately 24 million people, or 8% of the entire U.S. population, have diabetes, as do 23% of people who are 60 or older, according to the Centers for Disease Control & Prevention.
Diabetes results from disruption of the biochemical pathway that controls glucose utilization. At least as early as 1980, researchers recognized that Alzheimer's patients also have problems with glucose utilization, says Brown Medical School neuroscientist Suzanne M. de la Monte. Investigation into that connection fell out of favor with the advent of the amyloid hypothesis, which attributes Alzheimer's disease to the toxic effects of amyloid and tau protein aggregates in the brain. But now, de la Monte says, researchers are taking a second, closer look at the link between glucose utilization and Alzheimer's disease. And this time around, they're extending that connection all the way to diabetes.
When the digestive system converts food into glucose and releases glucose into the bloodstream, cells throughout the body absorb the sugar as a source of energy. Some cells, including most of those in the brain, can take up glucose without external help. But most cells—including those in muscle, fat, and the liver—need help to absorb glucose, and that's where insulin comes in.
During digestion, beta cells in the pancreas ramp up their insulin production. This hormone binds to receptors on insulin-sensitive cells, triggering an increase in the number of glucose transporter proteins in the cells' membranes. These transporter proteins shuttle glucose from the bloodstream into the cells. After digestion finishes, insulin levels decline, both because production tapers off and because the insulin on hand gets metabolized.
This complex biochemical dynamic can break down in a variety of ways. For instance, the immune system can destroy the pancreatic beta cells that produce insulin. This autoimmune attack causes type 1 diabetes, usually in childhood or early adulthood. Those with this condition usually manage it with daily insulin injections.
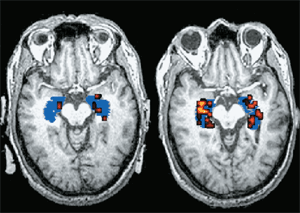
Type 2 diabetes begins when the body ceases to use insulin effectively. Central to this type of diabetes is a systemic resistance to insulin, which means that cells require more insulin than normal to respond to and absorb glucose. The pancreas tries to compensate by churning out extra insulin but ultimately is unable to keep up with the demand, and it eventually stops making insulin.
Type 2 diabetes accounts for 90–95% of diabetes cases. It used to show up primarily in older people but is increasingly afflicting younger ones as well. The incidence of this type of diabetes is rising largely as a result of the obesity epidemic, according to Griffin P. Rodgers, director of the National Institute of Diabetes & Digestive & Kidney Diseases.
"Prediabetics" with elevated blood glucose can lower their risk of advancing to type 2 diabetes if they lose weight and get more exercise. If they progress to full-blown type 2 diabetes, they can keep their disease in check through weight loss and exercise and by controlling blood pressure and cholesterol. Some type 2 diabetics use insulin injections or other medications to regulate blood glucose.
Although insulin's activity throughout much of the body is pretty well understood, its behavior in the brain is not. Even its source in the brain is uncertain. "People don't know exactly where the insulin in the brain is made or how it gets into the brain," Schubert says.
Jeffrey M. Burns, a neurologist who directs the Alzheimer & Memory Center at the University of Kansas Medical Center, believes that most insulin in the brain comes from the pancreas. However, Brown's de la Monte has shown that brain cells can produce insulin.

Wherever the insulin comes from, it's clear that the brain makes good use of it: Cell membranes at synapses, the junctions between neurons, are studded with insulin receptors. Yet many of these brain cells can absorb glucose without insulin's help. So why do neurons need insulin, and how does its activity go awry in diabetes and Alzheimer's?
A KEY HYPOTHESIS is that insulin promotes the growth and survival of neurons. Several findings back this idea.
For example, de la Monte determined that expression of the genes for insulin and its receptor, as well as an associated hormone known as insulin-like growth factor and its receptor, declines in the brains of people with Alzheimer's disease. As a result, she believes, their production of insulin wanes, and the response to the hormone in their brains decreases as the disease progresses.
Burns also found that the amount of insulin in the brains of Alzheimer's patients correlates with their mental capabilities; lower insulin levels are associated with more brain atrophy and poorer cognition (Neurology 2007, 69, 1094).
And Northwestern University neurobiologist William L. Klein has found evidence that binding of insulin to its receptors in the brain is crucial for supporting healthy synapses, which are essential for learning and memory.
They may agree that insulin plays important roles in the brain, but each of these three scientists has a different view on the biochemical mechanism by which a decline in insulin and the brain's response to the hormone affects cognition.
As de la Monte sees it, these changes impair the ability of brain cells to use glucose as a fuel to aid the biochemical processes that underlie learning and memory, including formation and maintenance of synapses. She says the glucose deprivation leads to oxidative stress; DNA damage; amyloid deposition; and loss of choline acetyltransferase, an enzyme that produces the neurotransmitter acetylcholine, which is important for cognition.
Burns, on the other hand, thinks reduced insulin signaling leads to over-activity of several neuronal proteins, with attendant harmful consequences for the brain. One example is glycogen synthase kinase-3β, which phosphorylates tau. Excess activity of this protein generates toxic hyperphosphorylated tau that accumulates into "neurofibrillary tangles" in the brains of Alzheimer's patients.
Meanwhile, Klein is focused on the involvement of insulin receptors in what he terms a "synaptic struggle for survival." Their foe: soluble amyloid-β peptide oligomers, which Klein and colleagues discovered in 1998 and refer to as amyloid-β-derived diffusible ligands, or ADDLs. These toxic molecules accumulate in the brain of Alzheimer's patients and have been linked to destruction of synapses and subsequent memory loss.
Klein has found that binding of ADDLs to a neuron's synapses draws insulin receptors from the cell's membrane into its center, where the receptors can't be accessed by insulin. If this process goes far enough, the neurons become less responsive to insulin.
Klein says his team recently showed in cultured brain cells that "insulin doesn't take this lying down." Binding of insulin to its own receptor activates a biochemical pathway that removes ADDL receptors from the surface of neurons (Proc. Natl. Acad. Sci. USA 2009, 106, 1971). However, the fight against ADDLs becomes progressively harder to win. That's because aging and diabetes diminish insulin signaling in the brain, Klein says. The outcome of the battle determines whether a person's memory becomes impaired.
OTHER RESEARCHERS are concentrating more on the biochemical effects of glucose than of insulin on cognition. Schubert, for example, is studying the effects of diabetics' high blood glucose in blood vessels in the brain.
Through a process known as glycation, the excess glucose binds to proteins in the blood and impairs their normal function, Schubert notes. Diabetics also produce a considerable amount of methylglyoxal, a metabolic by-product of glucose that's even better than the sugar at glycating proteins to form "advanced glycation end products" (AGEs).
"Methylglyoxal causes proteins to aggregate and to crosslink and become inactive," Schubert says, adding that "this type of modification is very abundant in Alzheimer's brain and in diabetic tissue." He believes the toxic AGEs help amyloid-β—the peptide that's the hallmark of Alzheimer's disease—damage blood vessels in the brain. "I think this is one of the major links between high glucose, diabetes, and Alzheimer's disease," he says.
Schubert's group recently studied the impact of AGEs in mice that have a genetic predisposition for developing Alzheimer's. The researchers induced some of these animals to acquire diabetes at a young age by destroying their insulin-producing cells. As time passed, more AGE deposits formed in the brains of the diabetic mice than the nondiabetic mice, and the diabetic mice developed Alzheimer's earlier (Neurobiol. Aging, DOI: 10.1016/j.neurobiolaging.2008.02.010).
This finding has serious implications if it can be extrapolated to humans. Because of the epidemic of childhood obesity, people are getting type 2 diabetes at ever-younger ages. As a result, Schubert says, they are increasingly at risk for developing Alzheimer's earlier in their lives.
So what's the bottom line on the link between Alzheimer's and diabetes? De la Monte takes the most radical stance on that question. "Alzheimer's disease and type 2 diabetes affect different parts of the body, but they are manifestations of the same disease," she contends. Indeed, she refers to Alzheimer's as type 3 diabetes.
She also includes nonalcoholic steatohepatitis (NASH) in the same group of diseases. NASH, which primarily affects the liver, is characterized by insulin resistance, inflammation, and fat accumulation in the organ. The condition can also lead to cirrhosis. Many NASH patients have diabetes, and many diabetics have NASH, de la Monte says. "So we have overlap of the same disease process, but affecting different parts of the body."
The concept of one type of disease producing symptoms in multiple organs "is not entirely new," de la Monte adds, noting that atherosclerosis affects the kidneys, the brain, and the heart, yet those conditions aren't thought of as three different diseases.
If diabetes and Alzheimer's are indeed two different facets of the same disease, then Alzheimer's could conceivably be treated in a way similar to diabetes.
One approach is to use insulin itself. Both Burns and Suzanne Craft, a University of Washington professor of psychiatry and behavioral sciences, are evaluating the hormone as a treatment for cognitive impairment in Alzheimer's patients.
Injecting nondiabetics with insulin isn't practical because the hormone would cause undesirable side effects such as driving down their blood sugar, Burns says. But "if you spray insulin into the nose, it crosses the blood-brain barrier and gets into the brain without affecting the body's blood sugar status."
Craft has shown that intranasal insulin improves memory in patients with mild cognitive impairment or early-stage Alzheimer's (Neurology 2008, 70, 440). Craft's group and Burns's own team are studying the brain mechanisms behind that observation.
Advertisement
But insulin may have limited utility because brain cells in Alzheimer's patients gradually become less responsive to the chemical, de la Monte cautions. Once that happens, "high levels of insulin are not necessarily of any benefit," she says. "They may even be harmful."
ANOTHER APPROACH is to normalize insulin function with "insulin sensitizing" drugs such as rosiglitazone and pioglitazone. These thiazolidinedione compounds, which are already available for treating diabetes, have shown some memory benefits for Alzheimer's patients in clinical trials, Burns says.
The compounds have anti-inflammatory properties, improve insulin activity, and also help insulin protect neurons against damage by ADDLs. The drugs work by activating peroxisome proliferator-activated receptors (PPARs), which control the expression of genes that are normally regulated by insulin. However, the drugs activate the gamma version of the receptor, which is most common in muscle and fat tissue, de la Monte says. Most PPARs in the brain are the delta type of receptor, so companies are now developing compounds specific for delta PPARs, she notes.
If such a compound can be developed, Northwestern's Klein thinks it would need to be paired with a treatment that reduces the load of toxic ADDLs in the brain. Vaccines and therapeutic antibodies are under development for this purpose, he says.
Schubert says successful treatment will require changes in diet and lifestyle as well as the use of drugs with multiple targets. One potential source for such a drug is curcumin, the polyphenolic yellow-orange curry spice derived from the turmeric plant. Schubert's group is working on a synthetically modified form of curcumin that inhibits glycation and also shows anti-inflammatory activity. The modified curcumin shows promise for treating both Alzheimer's and diabetes.
For now, de la Monte recommends exercise to increase blood flow to the brain and to increase insulin responsiveness throughout the body. That won't prevent Alzheimer's, she admits, but it will help you to have a clearer mind. "You're making the brain work harder," she says, "and you're delivering more nutrients to the brain."





Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter