Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Research Integrity
Guiding Stem Cell Research
NIH finalizes steps to ensure ethical and responsible work
by Britt E. Erickson
July 13, 2009
| A version of this story appeared in
Volume 87, Issue 28
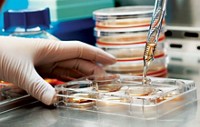
The National Institutes of Health has released its final guidelines for work on human embryonic stem cells (hESCs), opening the door for more stem cell lines to be eligible for federally funded research. The guidelines, which went into effect on July 7, address concerns about preexisting cell lines and establish a registry that will list all approved hESCs.
According to the new guidelines, hESCs that were eligible for federally funded research under the Bush Administration's policy will not automatically be eligible now. Instead, researchers must submit documents to NIH showing that the cells were derived responsibly. A special working group will determine whether the principles of informed consent—by embryo donors—outlined in the guidelines were sufficiently met. Cell lines derived on or after July 7, however, will have to follow specific requirements regarding informed consent by embryo donors. All hESCs that are approved will be posted on a new NIH registry.
In developing the guidelines, NIH considered more than 49,000 public comments, according to NIH Acting Director Raynard S. Kington. "We believe these guidelines are responsive to the public comments, will greatly expand our opportunities for stem cell research, and will ensure that NIH-funded research using human embryonic stem cells will be conducted in an ethical and responsible way," Kington said during a telebriefing.
Consistent with draft guidelines issued in April (C&EN, April 27, page 6), research on hESCs derived from leftover embryos created for reproductive purposes will be eligible for federal support, but research on hESCs derived from all other sources will have to be funded through private sources.
Some scientists are disappointed that the guidelines exclude research on hESCs created through a cloning technique called somatic cell nuclear transfer or an experimental procedure called parthenogenesis, in which stem cells are derived from an unfertilized human egg. But they are optimistic that such methods will be accepted in the future once public awareness of them grows.
"We were never dependent on federal funding for any of our therapeutic research," notes Kenneth C. Aldrich, chairman, CEO, and cofounder of International Stem Cell Corp., which pioneered parthenogenesis. "As time goes on, however, it will become increasingly obvious that there is a need for independent researchers who are trying to take our technology and put it to work to get some federal funding," he says.
Reps. Diana DeGette (D-Colo.) and Michael Castle (R-Del.) are pleased with the guidelines but remain committed to expanding them through legislation that "will promote all ethical forms of stem cell research." DeGette and Castle cosponsored legislation to expand federal funding of stem cell research that former president George W. Bush vetoed twice.
NIH developed the guidelines under an executive order President Barack Obama signed in March, which lifted restrictions on federal funding for hESC research that the Bush Administration put in place (C&EN, March 16, page 7).



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter