Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Halogen Bonding Begins To Fly
ACS Meeting News: Noncovalent interaction akin to hydrogen bonding is becoming an important tool for chemists
by Stephen K. Ritter
September 21, 2009
| A version of this story appeared in
Volume 87, Issue 38

Hydrogen bonding has evolved from being a curious intermolecular interaction between a hydrogen atom and an electron-rich partner to being a powerful tool for crystal engineering of solid-state materials and for molecular control of protein and DNA chemistry. Now, chemists are making progress in understanding and harnessing halogen bonding, a similar phenomenon pairing a halogen atom with an electron-rich partner.
The nature of halogen bonding and its practical applications were discussed at last month's ACS national meeting in Washington, D.C., during a symposium cosponsored by the Divisions of Organic Chemistry and Fluorine Chemistry on "Halogen Bonding: A World Parallel to Hydrogen Bonding." According to symposium co-organizers Pierangelo Metrangolo and Giuseppe Resnati of Polytecnico di Milano, in Italy, the event was the first international meeting fully devoted to halogen bonding.
The aim of the symposium was to "draw the attention of the global scientific community to the huge and largely unexplored potential of halogen bonding by presenting its basic concepts and identifying and defining the boundaries of the field with a selection of the most interesting applications," Metrangolo told C&EN.
"This recognition is helping halogen bonding grow out of its infancy and become a major topic in the chemical sciences," Resnati added.
Like hydrogen bonding, halogen bonding is a noncovalent electrostatic interaction between an electron-poor Lewis acid and an electron-rich Lewis base. In hydrogen bonding, hydrogen acts as the Lewis acid and interacts with atoms bearing a spare pair of electrons, such as nitrogen, oxygen, and halogens. The distance and strength of the interaction fall short of a formal covalent bond, but they're sufficient to orient and provide structure to the participating molecules.
In halogen bonding, a halogen plays the role of electron acceptor to donors such as nitrogen, oxygen, and even anions such as halides. At first blush, the electronegativity of halogens makes it seem improbable that halogen bonding could exist. But when halogens are bound to an electron-withdrawing substrate, they can harbor electropositive character and be on the receiving end of an intermolecular interaction. Iodine is the most common member of the group to form halogen bonds, and it typically forms the strongest halogen bonds (I > Br > Cl > F).
Halogen bonding has been known to chemists for nearly 150 years and was explored briefly by chemists in the 1950s, Resnati pointed out. But the scientific community started to appreciate halogen bonding only in the past 15 years, he noted. Pioneering modeling and computational studies on hydrogen and halogen bonding by Anthony C. Legon of the University of Bristol, in England, helped get things started, Resnati said.
At the ACS meeting, Legon described his group's studies on the gas-phase rotational spectra of Lewis acid-base complexes. By systematically varying the base and then the acid, Legon explained, his team determined details of hydrogen bonding, such as the separation and relative orientation of the donor-acceptor atoms and, thus, the strength of their interaction. Legon used the same approach to study halogen bonds, but he switched to dihalogen molecules, such as F2, ClF, Cl2, BrCl, Br2, and ICl, as the acceptor molecules. "We found that the generalizations established for the hydrogen bond also apply to the halogenated version," he noted.
Halogen bonds tend to be slightly stronger than hydrogen bonds, Legon pointed out. And because the hydrogen atom is nearly uniformly electropositive, it can form hydrogen bonds with different bond angles as needed to match the orientation of the donor's molecular orbitals. In halogen bonding, however, the halogen has both electropositive and electronegative regions, which restricts halogen bonds to being linear relative to the halogen, the donor atom, and their covalently bonded substrates.
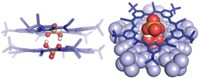
Peter A. Politzer of the University of New Orleans explained this phenomenon visually by showing maps of electrostatic potentials on the surfaces of the molecular orbitals of simple halogenated molecules. Under the influence of an electron-withdrawing substrate, a halogen atom can develop a region of low electron density on its outermost, exposed tip, giving rise to a partial positive charge, he said. Because this region is oriented along the axis of the bond between the halogen and its substrate, a halogen bond that forms lines up as a linear extension from the tip of the halogen atom.
The rest of the halogen atom has a partial negative charge, and it could interact with positive sites on other molecules, Politzer added. "This observation points out that it can be misleading to assign a single global charge to an atom in a molecule," Politzer said.

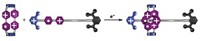
As Legon, Politzer, and other scientists have been exploring the physical attributes of halogen bonding, Metrangolo and Resnati's group has been using the phenomenon as an alternative to hydrogen bonding and metal-ion coordination in crystal engineering applications. The researchers found early on that halogen bonding between amines or imines and iodoperfluorobenzenes or iodoperfluoroalkanes is a useful directing force for constructing three-dimensional host-guest solids. In particular, they have shown that the N···I bond is one of the strongest and most versatile types of halogen bonds. This finding has set the stage for an initial round of novel developments.
One of those developments is in the world of liquid crystals. These materials, commonly used in electronic displays, rely on hydrogen bonding and aromatic-ring-stacking interactions for crystalline order. In 2004, Duncan W. Bruce of the University of York, in England, collaborated with Legon on the first reported use of halogen bonding to make liquid crystals. They showed that N···I halogen bonds between long-chain alkoxy-substituted stilbazoles and iodoperfluorobenzene orient the molecules to produce dimeric liquid crystals.
At the ACS meeting, Bruce presented his latest research exploring new combinations of compounds for making various types of liquid crystals. In one example, Bruce's group made a trimeric liquid crystal by sandwiching 4-iodotetrafluorophenol between two alkoxy-substituted stilbazoles. This unique combination forms a halogen bond via iodine on one side of the phenol ring and a hydrogen bond via a hydroxyl group on the other. Bruce anticipates that mixing and matching intermolecular interactions like that will lead to novel types of liquid-crystalline materials.
On a different application front, Metrangolo described a patent-pending method that he, Resnati, and coworkers devised to use halogen-bonded cocrystals as a means to separate and purify mixtures of diiodoperfluoroalkanes (DIPFAs). These compounds are produced on an industrial scale as monomers for a wide range of fluoropolymer products.
DIPFAs are typically made by telomerization, a process of building up perfluorocarbon chains from tetrafluoroethylene, Metrangolo explained. The resulting mixture of C2 to C12 species must be separated and purified by fractional distillation, he noted, which is an energy-intensive process and is limited to the volatile C8 and shorter compounds. To find an improved method to separate the entire mixture, the Italian team used bis(trimethylammonium) alkane diiodides of different chain lengths as host molecules with which to cocrystallize DIPFAs of matching chain lengths.

This "size-matching" allows a DIPFA to just fit into a void space in the ammonium compound's nonporous crystal lattice, where I···I halogen bonds hold the molecule in place, Metrangolo said. DIPFAs too small for the space fall out, and those too large simply can't enter. Heating the cocrystals liberates the purified DIPFA. Metrangolo said that an array of cocrystals with size-matched void spaces could be used like a filter to separate the mixtures.
Another fruitful area for halogen bonding is the development of solid-state materials for electronics applications. Hiroshi M. Yamamoto of RIKEN's Condensed Molecular Materials Laboratory, in Saitama, Japan, provided an overview of the use of halogen bonding to control the properties of organic semiconductors, metals, and superconductors. One project he is particularly excited about is constructing supramolecular insulating sheaths to separate conducting organic nanowires from each other.
Yamamoto and colleagues used stacks of planar ethylenedithiotetrathiafulvalene radical cations as the nanowire. The researchers then surrounded the wire with bromine and iodine counterions and tetraiodoethylene. The counterions serve as a halogen bond clamp to hold the insulating tetraiodoethylene molecules against the conducting core via Br···I and I···I bonds. The insulating ability of the supramolecular sheath is similar to that of epoxy resins commonly used in circuit boards, he added.
Yamamoto believes that controlling conducting properties by constructing an insulating network is a key development. "Scientists tend to pay the most attention to the conducting molecules in their development of conducting materials," he said. "But I find that the insulating moiety can play just as big a role when you consider an application."
Halogen bonding also holds vast potential for novel biomedical applications. Patrick Y. S. Lam of Bristol-Myers Squibb (BMS) related efforts to use halogen-bonding interactions in rational drug design. Medicinal chemists, he said, typically utilize the three classical interactions—hydrogen bonding, Coulombic interactions between charged groups, and hydrophobic interactions. But when BMS scientists were optimizing a potential anticoagulant drug a decade ago, halogen bonding was just gaining recognition. The team decided to give it a try as a parallel approach in the discovery pathway that led to the oral anticoagulant apixaban, which is now in Phase III clinical trials.
Lam and coworkers were designing inhibitors of Factor Xa, an important protease enzyme in the blood coagulation biochemical pathway. Inhibiting Factor Xa prevents blood clots, and it has emerged as a promising target for preventing and treating blood-clot-related diseases, Lam noted.
An initial drug screen identified a family of benzamidines as targets, Lam said. "These compounds are super binders but suffer from poor permeability and thus poor oral bioavailability," he added. The team used iodine to replace the amidine functional group to take advantage of a halogen-bond interaction with a key aspartic acid residue on Factor Xa. Although the iodine analog ultimately did not become apixaban, "to the best of our knowledge, this is the first successful utilization of halogen bonding in rational drug design," Lam said.
Relating another biomedical example, Colorado State University's P. Shing Ho talked about engineering halogen-bonding capability into biomolecules. In one case, Ho and coworkers exchanged a brominated uracil for thymine in a Holliday junction. This complex of four overlapping DNA strands plays an important role in DNA repair and in introducing genetic changes into target organisms.
The substitution sets up a halogen bond opportunity on one DNA strand between the bromine and a phosphate oxygen. That potential halogen bond directly competes with a potential hydrogen bond on an adjacent DNA strand. Because the structure of the complex allows only one of the competing bonds to form at a time, if the bonds alternate, the DNA junction switches between two isomeric forms.
"This study was the first to show that halogen bonding can be used to construct molecular materials based on DNA or other biomacromolecules," Ho said. The ability to direct the conformation of DNA and proteins should improve the prospects for designing and controlling biomolecule-based nanodevices, he noted.
As Metrangolo, Resnati, and their colleagues continue to champion halogen bonding as a complement to hydrogen bonding, diverse possibilities in separation science, liquid-crystal displays, electronic materials, and biomedical applications will continue to grow, said Nancy S. Goroff of the State University of New York, Stony Brook. She uses halogen bonding as a scaffold to align monomer molecules in solid-state polymerization reactions. "Halogen bonding is not as ubiquitous as hydrogen bonding," she admitted. "But this symposium demonstrated nicely that it is a general phenomenon that can be exploited by chemists in many different ways."




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter