Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Tagging Sulfenic Acids
New probe leads to new insights in kinase redox biology
by Carmen Drahl
December 12, 2011
| A version of this story appeared in
Volume 89, Issue 50
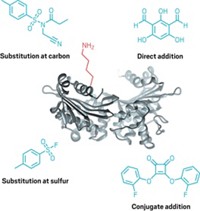
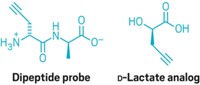
By tweaking a chemical probe, chemists have learned that a protein modification called sulfenylation influences signaling in the epidermal growth factor receptor (EGFR), a kinase enzyme implicated in multiple cancers (Nat. Chem. Biol., DOI: 10.1038/nchembio.736). Sulfenylation, or formation of sulfenic acid (–SOH) groups, can occur if a cysteine amino acid is exposed to an oxidant such as hydrogen peroxide. Tracking occurrences of the reaction and determining their implications in living cells remain challenging because established probes lack sensitivity or cell permeability. To make their latest probe, Kate S. Carroll of Scripps Florida and coworkers replaced the azide in a previous version with an alkyne. The probe turned out to be sensitive enough to detect differences in sulfenylation rates among various proteins in human cells. The team showed that sulfenylation of a specific active-site cysteine in EGFR, Cys797, enhanced its kinase activity. That cysteine is the target of several covalent drugs under development, but the drugs are designed to latch onto the cysteine in its thiol form. This development raises interesting questions about how to design irreversible inhibitors that target amino acids subject to redox modifications such as sulfenylation, Carroll says.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter