Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Hunt For The Male Contraceptive Pill Continues Despite Decades Of Research
Scientists search for new targets that could lead to a drug that can reversibly derail sperm production
by Michael Torrice
September 24, 2012
| A version of this story appeared in
Volume 90, Issue 39

When men and women plan their personal birth control strategies, men scan a much smaller menu of options than women do. A woman can pick from two types of intrauterine devices (IUDs), several forms of hormonal contraception such as the pill, multiple barrier methods such as diaphragms, or two sterilization procedures. A man can choose between a condom or a vasectomy.
Of course, vasectomies are not for every man, because they’re essentially irreversible. Meanwhile, condoms fail between 2 and 18% of the time, making them relatively less effective than many of the options for women.
This lack of options for men puts much of the birth control burden on women, says Debra J. Wolgemuth, a biologist at Columbia University Medical Center. “It should be a two-way street.” She and experts in reproductive medicine think that men could gain much-needed options through drugs that are as effective, safe, and reversible as the female birth control pill.
More male contraceptive options also could help prevent the large number of unintended pregnancies in the U.S., says John K. Amory, a doctor at the University of Washington, Seattle. About half of all U.S. pregnancies each year are unintended, and about 40% of those end in abortion, according to the Guttmacher Institute, a nonprofit organization that promotes reproductive health. Economists at the institute estimate that unintended pregnancies cost U.S. federal and state government programs about $11 billion every year.
Despite decades of research on male contraceptives, no treatment has moved past clinical trials to reach the market. And in the past five years, the few pharmaceutical players working on male contraception have mothballed their programs. Experts say part of the problem is that, for several reasons, the normal hurdles drugs face—achieving high efficacy and low toxicity—are even higher for male contraceptives. Researchers in academia and nonprofit organizations hope that new formulations of hormonal drugs or small molecules that hit nonhormonal targets in the testis could overcome these barriers.
During the early 2000s, there was a lot of excitement in the male contraceptive field, says Douglas S. Colvard, the deputy director of programs at CONRAD, a nonprofit organization that works on reproductive health research. In 2002, two pharmaceutical companies, Schering and Organon, teamed up to work on a hormonal method similar to the one women use. But the collaboration was short-lived; it ended in 2006 because Schering thought that the required multiple injections would deter men from using the method. Schering’s program completely stopped in 2007 after Bayer acquired the company. Organon’s work had terminated by 2009 when Merck bought Schering-Plough, which acquired Organon in 2007.
Experts in the field think pharmaceutical companies have shut down male contraceptive programs because the companies would rather spend resources on drugs that have a better chance of approval. New male and female contraceptives, experts say, face much tougher scrutiny in terms of safety and efficacy when compared with drugs that treat diseases such as cancer or heart disease.
In general, most drugs in development fail because of safety issues. A male birth control pill would face even higher safety standards, says Diana L. Blithe of the National Institute of Child Health & Human Development (NICHD). The side effects for such a drug would have to be negligible, because men would take the drug for long periods of time, she says. And unlike most drugs, a male pill would be prescribed to healthy people. “It’s not like with a cancer drug, where the person has a life-threatening illness and is willing to tolerate potential side effects.”
Blithe, who runs NICHD’s Male Contraceptive Development Program, also points out that when a contraceptive fails, the result is unplanned pregnancy. So any male drug would need to meet a high bar of efficacy before approval.
Most experts in the field agree that the standard would be a 1% failure rate, similar to that of female hormonal contraceptives. But unlike the female pill, a male drug faces a much tougher biological task. “Women make one egg a month,” says Amory. “Men make a thousand sperm every second, from the time they go through puberty until the day they die.”
This relentless sperm production, called spermatogenesis, happens inside coiled hoses in the testes. The process starts with regenerating stem cells called spermatogonia along the outer edge of these tubes. These stem cells differentiate and then go through meiosis, a special two rounds of cell division that produces cells with one set of each chromosome, instead of two. These resulting spermatid cells then mature into sperm cells with small heads and whipping tails. The whole process takes about 64 days.
Complex hormonal circuitry keeps this specialized assembly line moving. Spermatogenesis requires high levels of testosterone inside the testis. A hormone secreted by the pituitary gland called luteinizing hormone (LH) stimulates cells in the testis to pump out testosterone. Another pituitary hormone, follicle-stimulating hormone (FSH), turns on pathways in testis cells that launch meiosis and help sperm cells mature.
Because of this dependence on hormones, by far the most researched strategy to disrupt spermatogenesis is to follow the lead of female contraception and knock down levels of LH and FSH. In fact, the most effective male hormonal methods involve a combination of testosterone and a progestin, a type of synthetic hormone used in the female pill. Both hormones activate negative feedback pathways that tell the pituitary to release less LH and FSH.
Despite decades of research on hormonal methods, experts say most fail to clear the high efficacy and safety bars for male contraceptives. Amory points out that with the best hormonal methods, contraceptive failure rates still struggle to dip below 5%. What’s more, testosterone in these formulations can lead to undesirable side effects. In April 2011, a clinical study run by CONRAD and the World Health Organization ended early because of concerns over mood changes, such as irritability and depression, in participants.
Amory and other researchers think the way to circumvent the side effect issue is to target nonhormonal pathways specific to spermatogenesis. Their rationale is that hormones not only affect the testis, but also regulate pathways elsewhere in the body. Knocking down testis-specific targets, he says, could keep a drug’s effects focused on sperm production.
Over the past five years, researchers have published several drug leads for nonhormonal targets. These compounds cause complete and reversible infertility in animal models, with low toxicity.
Two of the molecules disrupt vitamin A’s regulation of spermatogenesis. Biologists have long known that men with vitamin A deficiency are sterile.
Columbia’s Wolgemuth identified one drug lead as part of her work on understanding the genetic pathways controlling spermatogenesis. One gene she studied codes for a transcription factor called retinoic acid receptor α (RARα). The protein binds a vitamin A metabolite called retinoic acid and regulates the expression of genes. Mice with the RARα gene knocked out “are perfectly healthy, except the male mice are sterile,” Wolgemuth says.
This testis-specific effect led Wolgemuth’s group to study a RARα inhibitor synthesized by Bristol-Myers Squibb. Last year, her team reported that the compound, BMS-189453, inhibits sperm production, in part by disrupting a late event in spermatogenesis (Endocrinology, DOI: 10.1210/en.2010-0941). During this stage, maturing spermatids must line up along the edge of tubes in the testis for release. The compound “royally messes that up, so they’re not aligned properly and they can’t get out,” Wolgemuth says. She and her colleagues are now looking for analogs of BMS-189453 that are more specific for RARα.
Meanwhile, Amory and his colleagues have looked at a target upstream of RARα, an enzyme called aldehyde dehydrogenase 1a2 (ALDH1a2). The enzyme helps convert vitamin A into retinoic acid.
In a paper published last year, they showed that a compound called WIN 18,446 halts spermatogenesis by inhibiting retinoic acid production by ALDH1a2 (J. Androl., DOI: 10.2164/jandrol.110.010751). On the basis of their findings, Amory and his colleagues are working on developing ALDH1a2-selective inhibitors as leads for contraceptives.
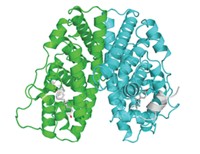
The most recent nonhormonal drug lead is JQ1, which inhibits a protein called BRDT. This protein regulates gene expression through two special protein regions, called bromodomains, that bind histones, the proteins that chromosomal DNA wraps itself around.
Wolgemuth’s group studied mice lacking one bromodomain in BRDT and discovered that the males were infertile. Other researchers had found that some infertile men have mutations in the BRDT gene.
On the basis of these data, James E. Bradner of Dana-Farber Cancer Institute and Martin M. Matzuk of Baylor College of Medicine thought that BRDT might regulate genes important for spermatogenesis. They and their colleagues picked JQ1 as a possible BRDT inhibitor because the compound came from a library of anticancer compounds targeting another bromodomain protein called BRD4. Through biochemical studies and X-ray crystallography, they found that JQ1 blocks BRDT from binding histone proteins.
In a paper published last month, the scientists showed that JQ1 causes reversible infertility in male mice (Cell, DOI: 10.1016/j.cell.2012.06.045). Now Bradner and Matzuk plan to use JQ1 as a lead compound to produce a second generation of compounds that are specific to BRDT.
The nonhormonal compound closest to clinical trials is H2-gamendazole. Gunda I. Georg of the University of Minnesota, Twin Cities, and Joseph S. Tash of the University of Kansas Medical Center developed the compound without a specific protein target in mind. They started with a compound called lonidamine, an anticancer molecule developed in the 1970s. Data on the compound showed it halts spermatogenesis in several types of animals. But the molecule also is toxic to the liver and kidneys. So they set out to make analogs of the compound that blocks sperm production but has no toxicity issues.
Georg’s lab would supply Tash’s group with analogs, and Tash’s researchers would screen them in rats, Tash says. From these screens, they found gamendazole. In a 2008 study, the team showed that a single oral dose of the compound makes male rats infertile after three weeks (Biol. Reprod., DOI: 10.1095/biolreprod.106.057810). Through further tests, the researchers found that gamendazole disrupts the close relationship between developing sperm cells and another type of cell, called a Sertoli cell. These cells transport and nourish sperm cells as they mature. Without this nursing, spermatids release prematurely from the tubules before they can become mature sperm cells, Tash says.
Since the 2008 study, the researchers have started testing a related compound, H2-gamendazole. (To make it, they reduced the carbon-carbon double bond adjacent to gamendazole’s carboxylic acid.) Tests in several types of animals, including rabbits and monkeys, show the compound doesn’t have any of the toxicity issues that lonidamine has. Tash and Georg think the compound is almost ready for preclinical safety tests and hope to discuss testing with the Food & Drug Administration.
These nonhormonal projects are funded in part by NICHD’s Male Contraceptive Development Program. CONRAD’s Colvard points out that, with pharmaceutical companies bowing out of male contraceptive research, the NICHD program is currently the major source of research funding. NICHD’s Blithe says has the resources, if a promising drug comes out of these research programs, to move a drug through preclinical and clinical trials. Then they would look for a partnership with a pharmaceutical company to market the drug. For a nonhormonal drug, that process could take more than a decade. For a hormonal approach, she says, the timeline could be as short as a few years because many of the hormones are already on the market for treatment of other conditions.
Advertisement
So how long will it be before men can have their own birth control pill? Amory jokes that people in the field have been promising a drug in the next five to 10 years for the past 30 years. He thinks a decade sounds about right, and most scientists working on nonhormonal methods agree.
In the end, Blithe says, the path to the first male contraceptive drug will be the toughest: “Once the first one gets out there and companies see that it is a viable, lucrative product, there will be more effort put into the next one.”




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter