Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
RNA Sugars May Have Had Cyanide Origins
Study lends further support to RNA’s role in the origin of life on Earth
by Carmen Drahl
October 1, 2012
| A version of this story appeared in
Volume 90, Issue 40
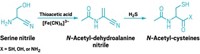
The primeval recipe for RNA’s sugar components likely started with hydrogen cyanide, not formaldehyde as researchers had thought (Nat. Chem., DOI: 10.1038/nchem.1467). John D. Sutherland of the Medical Research Council Laboratory of Molecular Biology, in Cambridge, England, previously reported a route to ribonucleotides that would be plausible on an early Earth, supporting RNA’s involvement in the origin of life. But his team didn’t show how RNA’s sugar building blocks—glycolaldehyde and glyceraldehyde—could arise from one-carbon precursors. Most chemists cite the formose reaction, an oligomerization of formaldehyde, as the source of prebiotic sugars. But that reaction yields messy product mixtures. Sutherland and postdoc Dougal Ritson now suggest an alternative pathway inspired by a century-old sugar synthesis. They exposed an aqueous solution of hydrogen cyanide and copper cyanide to UV light and observed oxazolidinones as major products. Those compounds “are a smoking gun” for the sugars, Sutherland says. Cyanate formed in the reaction traps the sugars in their oxazolidinone forms as they are generated, he explains. The researchers are now trying to replace copper with iron, because iron’s redox properties should prevent cyanate formation, leading to free sugars.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter