Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Business
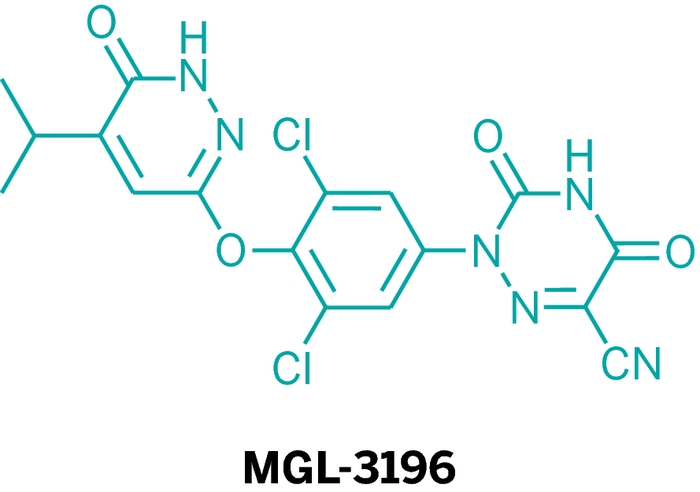
MGL-3196, Thyroid Hormone Analog
by Carmen Drahl
April 22, 2013
| A version of this story appeared in
Volume 91, Issue 16

No conversation about preventing heart disease would be complete without mentioning statins, the blockbuster cholesterol-lowering class of medications. Yet not all patients on statins reach the cholesterol-lowering goals their doctors recommend. Statins aren’t very good at managing other lipids linked to heart disease, such as triglycerides. And nearly one in five people taking statins experiences side effects, such as muscle weakness. Some experts see opportunities for new types of cholesterol-lowering drugs to gain a foothold.
That’s the spirit behind MGL-3196, an experimental medication discovered at Hoffmann-La Roche’s now-shuttered site in Nutley, N.J., and currently in development at Fort Washington, Pa.-based Madrigal Pharmaceuticals. Martha J. Kelly, Madrigal’s head of research and compound development, unveiled the molecule. MGL-3196 has a mode of action distinct from that of statins. Instead of preventing cholesterol synthesis, as statins do, MGL-3196 is designed to increase cholesterol uptake into the liver and increase its metabolism, and thereby its elimination from the body through the bile. The compound is also intended to boost fat metabolism, thereby reducing triglyceride levels.
MGL-3196
\
Company: Madrigal Pharmaceuticals
Target: thyroid hormone receptor-β, in the liver
Disease: high cholesterol, high triglycerides
Specifically, MGL-3196 is a thyroid hormone analog, designed to target a thyroid hormone receptor in the liver. These receptors regulate genes involved in cholesterol regulation and metabolism.
Thyroid hormone affects the body in many ways. Roche’s team sought the hormone’s beneficial metabolic effects while avoiding unwanted effects such as elevated heart rate and bone loss. The side effects tend to arise when activating the alpha form of the thyroid hormone receptor and when activating the receptor in tissues outside the liver. So the Roche team sought molecules that were targeted to the liver and were selective for thyroid hormone receptor-β.
Rather than screening for compounds by assessing only their binding to the target, Roche’s team used a screen that also assessed compounds’ activity. This helped them find molecules early on that were selective for the β-receptor. To replace the electron-rich phenols common on previous compounds, the chemists chose pyridazinones. (Phenols can confer poor metabolic properties.) Along the way, they noted the molecules reached the liver selectively and had poor penetration into the heart and other tissues. All this led to the drug candidate that today is known as MGL-3196.
But it didn’t get that name right away. In 2008, the molecule moved from Roche to San Francisco biotech VIA Pharmaceuticals along with Rebecca A. Taub, who left her position of vice president and head of metabolic disease research at Roche to become VIA’s senior vice president for research and development. Roche scientists at the time were focused not on cholesterol but on other metabolic disease areas, Taub told C&EN. The move made it possible to test the lipid-lowering activity, which Taub called the molecule’s “most profound effect.” Taub later cofounded Madrigal, which acquired the candidate and some other VIA assets in 2011.
In animal testing, MGL-3196 lowered levels of cholesterol and triglycerides without affecting high-density lipoprotein, or HDL (good cholesterol), or triggering side effects in the heart. It also appeared to work in combination with statins, lowering lipid levels more than would be expected by combining the molecules’ individual effects.
The molecule has completed Phase I clinical trials. To evaluate its safety and to obtain proof of concept of its effects on lipid levels, otherwise healthy volunteers with slightly high low-density lipoprotein (LDL) levels received various daily doses of the drug candidate or a placebo. In the two-week multiple-dose study, the drug appeared safe at all doses tested. Those receiving MGL-3196 had statistically significant reductions in LDL levels, and some statistical trends from the study suggest reductions in triglycerides as well.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter