Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Environment
Soap Under Scrutiny
Regulation: FDA proposes rule to require antibacterial soap makers to show product safety, effectiveness
by Britt E. Erickson
December 23, 2013
| A version of this story appeared in
Volume 91, Issue 51

A rule proposed by the Food & Drug Administration last week has left some chemical producers and others in the cleaning products industry perplexed. If the rule is finalized, manufacturers of antibacterial soaps and bodywashes will have to show that their products are safe for everyday use and that they are more effective than ordinary soap at preventing the spread of germs. Companies that fail to demonstrate such safety and effectiveness will have to either reformulate or relabel their products.
Two trade groups—the American Cleaning Institute and the Personal Care Products Council—argue that industry has already provided such data to FDA. According to them, dozens of studies have shown that hand washing with antibacterial soap “produces statistically greater reductions in bacteria on the skin than when using non-antibacterial soap.”
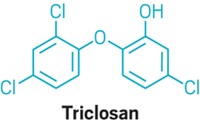
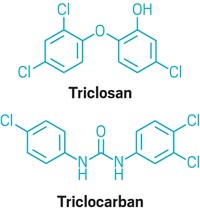
FDA took action because of growing concerns about possible health effects associated with antibacterial ingredients and their potential to lead to antibiotic resistance. Animal data suggest that daily exposure to some active ingredients used in antibacterial soaps—for example, triclosan and triclocarban—could result in hormonal effects, says Sandra L. Kweder, deputy director of FDA’s Office of New Drugs. “Laboratory studies also suggest that antibacterial ingredients may contribute to changes in antibiotic susceptibility,” she notes.
The trade groups contend that “the use of antibacterial wash products in the home environment does not contribute to antibiotic or antibacterial resistance.”
“Millions of Americans use antibacterial hand soaps and bodywashes,” Kweder says. “They are used every day at home, work, schools, and other public settings where the risk of bacterial infections is relatively low.” Kweder estimates that there are about 2,000 antibacterial soaps and bodywash products on the market today in the U.S.
Because consumers are exposed to high levels of these antibacterial ingredients, “there should be clearly demonstrated benefit from using antibacterial soaps to balance out any potential risk,” Kweder notes. “FDA does not have data to demonstrate that these products are any more effective at preventing people from getting sick than washing with plain soap and water,” she says.
FDA’s proposed rule is part of a settlement reached last month with the environmental group Natural Resources Defense Council (NRDC). The group sued FDA in 2010 for not finalizing a 1978 rule that would have banned triclosan from certain consumer products. Under the settlement, FDA committed to issuing the proposed rule and taking final action by 2016.
“This is a good first step toward getting unsafe triclosan off the market,” says Mae Wu, an attorney with NRDC’s health program. “Washing your hands with soap containing triclosan doesn’t make them cleaner than using regular soap and water and can carry potential health risks.”
Growing consumer pressure has already led some companies, including Johnson & Johnson and Procter & Gamble, to start phasing out the use of triclosan in their products. Others, such as Colgate-Palmolive, have reformulated some of their products so that they no longer contain triclosan and relabeled others so that they no longer make antibacterial claims.
The proposed rule does not apply to toothpaste, hand sanitizers, wipes, or antibacterial products used in health care settings or food preparation facilities. FDA is accepting comments on the rule through June 2014.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter