Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Redirecting Amyloid Fibril Growth
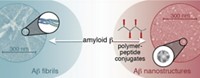
A polymer-peptide conjugate causes amyloid-ß peptide to assemble into discrete nanostructures instead of fibrils
by Journal News and Community
April 21, 2014
| A version of this story appeared in
Volume 92, Issue 16
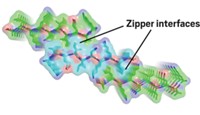
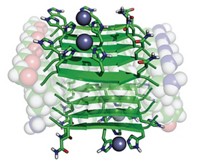
Synthetic macromolecules can redirect the aggregation of amyloid-β (Aβ), the peptide associated with brain plaques in Alzheimer’s disease, from microscale fibrils to spherical nanostructures, chemists report (J. Am. Chem. Soc. 2014, DOI: 10.1021/ja501102f). The findings could help illuminate how amyloid fibrils grow, as well as suggest new therapeutic strategies for the disease, the researchers say. Jeffrey S. Moore and colleagues at the University of Illinois, Urbana-Champaign, wanted to disrupt amyloid assembly by interfering with weak interactions between Aβ monomers. Their inhibitors consisted of a copolymer backbone decorated with 21 copies of a peptide that binds to a region of Aβ that interacts with other monomers. When the team added the polymer-peptide conjugate to an equal amount of Aβ peptide, the conjugate completely suppressed fibril formation. The chemists were surprised to find that instead Aβ formed nearly spherical structures about 30 nm in diameter. The researchers are currently investigating the composition of these nanostructures. Understanding their structures and how they form may suggest new ways to prevent or disrupt amyloid aggregation, Moore says.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter