Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Germanium Nanowires Made Simply
Electrochemical method offers simple route to nanowire arrays of prized material
by Mitch Jacoby
September 15, 2014
| A version of this story appeared in
Volume 92, Issue 37
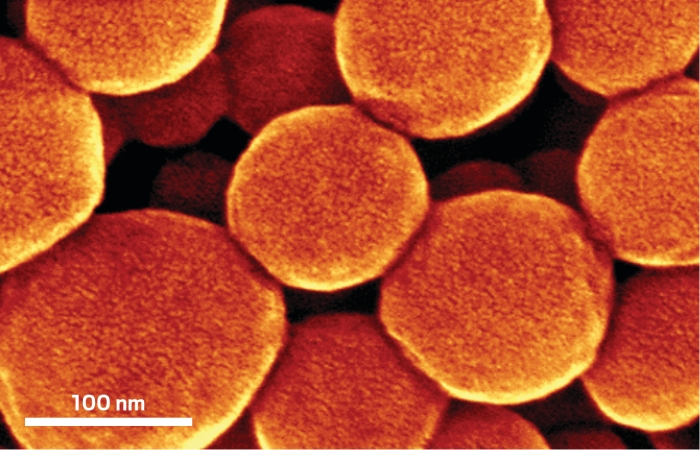
Germanium isn’t one of the more popular materials in high-tech gadgets, but it ought to be. It can conduct positive and negative charges through electronic devices better than silicon. And for lithium-ion battery use, germanium boasts a larger theoretical charge-discharge capacity than graphite, the standard anode material. But germanium is difficult and expensive to process. A team led by Jay A. Switzer of Missouri University of Science & Technology may have a simple solution. The team electrochemically reduces a layer of indium tin oxide sitting in contact with an aqueous Ge(IV) solution. That step decorates the underlying layer with evenly dispersed In particles, which serve as reduction sites for dissolved germanium species and as a medium in which germanium crystallizes. Continuous reduction and dissolution of germanium lead to saturation of germanium in indium and to growth of dense arrays of indium-tipped germanium nanowires (ACS Nano 2014, DOI: 10.1021/nn503784d). The high conductivity of the wires should make them ideal for Li-ion battery applications, the team suggests.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter