Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Physical Chemistry
Steroid Aromatase Dark Horse Wins
New study may finally resolve controversy over the mechanism of key androgen-estrogen conversion reaction
by Stu Borman
October 6, 2014
| A version of this story appeared in
Volume 92, Issue 40
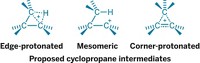
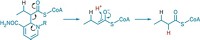
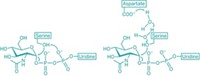
Enzyme specialists believe they have finally nailed down the controversial mechanism of a key mammalian hormone conversion process called the steroid aromatase reaction (J. Am. Chem. Soc. 2014, DOI: 10.1021/ja508185d). The conversion of androgens to estrogens maintains the proper balance of sex hormones in the body. And cytochrome P450 19A1, the iron-dependent aromatase enzyme that catalyzes the reaction, is a drug target for estrogen-dependent breast, uterine, and ovarian cancers. Researchers have therefore been trying to determine how the reaction works at a detailed molecular level. But the active iron species in the form of the enzyme involved in the third and final step of the reaction has been uncertain—no fewer than five mechanisms have been proposed. Some studies support an FeO3+ species called Compound I, but a more favored mechanism involves ferric peroxide (FeO2–) instead. Francis K. Yoshimoto and F. Peter Guengerich of Vanderbilt University School of Medicine have now used isotopic labeling to provide definitive evidence that Compound I is the active iron species in the third step, beating out the more favored proposal.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter