Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Bottle-Brush Polymer Mimics Natural Joint Lubricant
Biomaterials: The material could serve as a water-based lubricant in artificial joints, extending their lifetimes
by Leigh Krietsch Boerner
April 24, 2014

When doctors implant artificial joints in patients, they usually have to replace the devices after 10 years because the lack of good lubrication causes the joints to wear out. To find a better water-based lubricant, researchers now have synthesized a polymer that mimics the structure and function of lubricin, a protein that occurs naturally in the fluid that cushions our joints (J. Am. Chem. Soc. 2014, DOI: 10.1021/ja501770y).
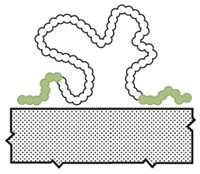
The key to lubricin’s lubricating power lies in its structure, says Xavier Banquy, a biomaterials chemist at the University of Montreal. The protein has two end domains that anchor it to surfaces, and a dense, but springy, area in the middle shaped like a bottle brush. When two lubricin-coated surfaces move toward each other, they don’t actually touch; instead the bottle-brush domains touch and compress as the applied pressure increases. The bottle-brush structure also allows water, an excellent lubricant, to flow through the material when the two surfaces are moving past each other. The protein lubricates effectively regardless of how much pressure is put on the two surfaces or how fast they move back and forth.
While working at the University of California, Santa Barbara, Banquy, his advisor Jacob Israelachvili, Krzysztof Matyjaszewski of Carnegie Mellon University, and coworkers developed a synthetic version of the lubricin structure. This material consists of a methyl methacrylate backbone running through the bottle brush and two end domains. In the bottle-brush block, chains of poly(2-methacryloyloxyethyl phosphorylcholine), a zwitterionic polymer, dangle off the backbone. The team synthesized these brush bristles using a technique called atom transfer radical polymerization. Because of the polar nature of zwitterionic side chains, the polymer can hold a lot of water, Banquy says. The two blocks flanking the bottle brush are decorated with a positively charged amine groups, which can bind strongly to the mica surfaces used by the group to test the lubricant. The researchers can easily change the groups decorating the flanking domains so that the polymer attaches to different types of surfaces, Banquy says.
To test the lubricant, the researchers placed the polymer between two mica plates and measured both the normal force by moving the plates together and the friction force by sliding them past each other. They then calculated the friction coefficient of the polymer. They found that their material lubricates much better than lubricin over a wide range of sliding speeds and pressures, Banquy says. The ability to produce low levels of friction even at high pressures is partially due to the material’s binding domains that help the lubricant stick strongly to the mica surfaces.
The use of polymer brushes as a lubricant is a novel idea, says S. Michael Kilbey, a materials chemist at the University of Tennessee, Knoxville. The density of the brush’s side chains allows the lubricant to resist compression, making it functional at high loads, he says.
The researchers are now working on oil-based lubricants using the same design principle, Banquy says. These types of lubricants may be more useful for industrial purposes.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter