Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
How Researchers Formed A Pure, Chiral Crystal From Mixed Ingredients
Stereochemistry: Team grows homochiral crystals despite expectations
by Stu Borman
November 18, 2015
| A version of this story appeared in
Volume 93, Issue 46

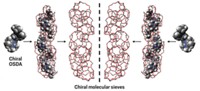
Scientists have long thought that chiral molecules could only form pure, orderly chiral crystals if those molecules all have the same chirality. Virgil Percec of the University of Pennsylvania and coworkers have now defied that long-established notion about the need for homochiral building blocks by growing high-purity supramolecular crystals from racemic and diastereomeric mixtures of perylene bisimides (PBIs) (Nat. Chem. 2015, DOI: 10.1038/nchem.2397).
In a few past studies, research groups had shown that enantiomerically mixed polymers or supramolecular assemblies could separate into or form enantioenriched domains or crystals, but the products were not chirally pure or highly ordered. The new crystals, on the other hand, form when mixtures of PBIs stack on top of one another, self-assembling into chiral supramolecular helices. These columnar structures then combine to form pure homochiral crystals.
“This is a very significant discovery challenging the accepted view that homochirality is an essential prerequisite for self-assembly to form high-quality homochiral supramolecular crystals,” says Boris Rybtchinski, an expert on organic self-assembly at the Weizmann Institute of Science. The findings suggest that advanced homochiral materials for organic light-emitting diodes, spintronics, and other applications “could be achieved in a cost-effective way, starting from readily available nonhomochiral mixtures instead of very expensive, pure enantiomers.”
Percec and his team propose that their PBIs form homochiral crystals because the columnar helices that initially form by self-assembly have alkyl chains around their outsides that interlock in a cogwheel-like manner with chains on neighboring helices, which go on to interlock with other helices. Each PBI has six stereocenters, which end up facing inward toward the central axes of the columnar helices. Those stereocenters therefore don’t interfere with the interlocking process, making their configurations inconsequential to crystal formation.
Although the researchers observed the phenomenon on just one series of PBIs, the proposed mechanism suggests that it might be possible to extend the concept to other nonhomochiral building blocks with similar molecular features, says Ricardo Riguera of the University of Santiago de Compostela, whose interests include controlled chirality.
Supramolecular systems specialist E. W. (Bert) Meijer of Eindhoven University of Technology calls the study “remarkable.” It shows “that mixtures of molecules with even six stereocenters and all possible chiralities always give one type of supramolecular product,” he says. “This is unexpected for many in the field. The results give new insights into the amplification of chirality in large supramolecular structures and could be important for understanding the origins of homochirality in nature.”



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter