Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Transcription activation complex analyzed in high-def
Researchers determine detailed crystal structure of a protein-DNA assembly that controls transcription of a specific gene
by Stu Borman
June 13, 2016
| A version of this story appeared in
Volume 94, Issue 24
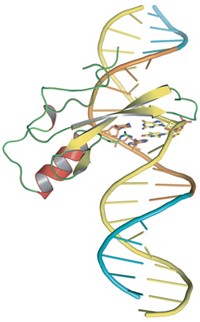
To better understand how DNA is transcribed into RNA, scientists have long been trying to obtain a detailed structure of a protein-DNA complex that initiates and regulates transcription of a specific gene. But such complexes have been hard to crystallize. Richard H. Ebright and coworkers at Rutgers University have now found a thermophilic bacterial complex that forms crystals readily and have determined its 4.4-Å structure (Science 2016, DOI: 10.1126/science.aaf4417). The complex includes a transcription activator protein, an initiation factor, RNA polymerase, a DNA template, and an RNA primer. The crystal structure reveals that a first set of protein-protein interactions between the activator and RNA polymerase helps the enzyme bind DNA and a second set of protein-protein interactions helps the enzyme unwind DNA so it can be transcribed. “It’s a lovely picture that you can tell is right” from decades of earlier biochemistry and genetics experiments on similar transcription activation complexes, comments transcription initiation expert Deborah M. Hinton of the National Institute of Diabetes & Digestive & Kidney Diseases.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter