Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Mutant enzyme provides a route to novel triterpenes
A single amino acid change leads to synthesis of new structures for important class of natural products
by Stu Borman
July 14, 2016
| A version of this story appeared in
Volume 94, Issue 29
Triterpenes are multiringed natural products useful as drugs, agrochemicals, and even cosmetics.
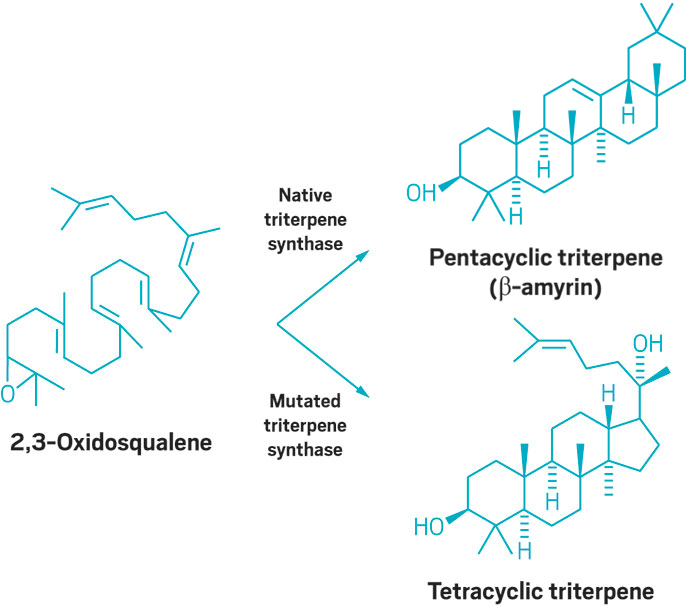
Researchers report that a single amino acid mutation in the enzymes that synthesize these compounds can produce novel products with four-ring triterpene core structures instead of the usual five-ring ones (Proc. Natl. Acad. Sci. USA 2016, DOI: 10.1073/pnas.1605509113). The modified enzymes could be engineered into microorganisms or plants to generate a variety of novel triterpenes that would be difficult or impossible to synthesize any other way.
The researchers, led by Anne Osbourn of the John Innes Centre, notes that their findings reveal “hidden functional diversity within triterpene synthases.” They hope to use the mutant enzymes in a “triterpene machine” that yields custom-built triterpenes.
Plants make triterpenes by using triterpene synthase enzymes to cyclize a linear substrate, 2,3-oxidosqualene, into pentacyclic natural products, such as the analgesic and anti-inflammatory agent β-amyrin. Other enzymes then further modify these triterpenes to produce a variety of derivatives, such as saponins, which are sugar-containing compounds that have been used as surfactants and vaccine adjuvants.
The key mutation for producing new triterpenes involved swapping a serine residue for a phenylalanine. When the team expressed the modified enzyme in yeast, they found that it could still cyclize the usual substrate, 2,3-oxidosqualene, but more readily catalyzed the cyclization of a more oxygenated substrate, dioxidosqualene. This yielded an unusual oxygen-enriched compound with a tetracyclic triterpene core structure.
Osbourn envisions developing a triterpene machine by inserting genes for the mutated enzymes into yeast or tobacco plants to enable the organisms to produce customized cyclic triterpenes cost-effectively and on a large scale.
“The novelty of the work is that the group is getting unique or unusual products that would not be readily available by conventional chemical synthesis, or any other means at the moment,” says Joseph Chappell of the University of Kentucky, an expert on the biosynthesis of terpene-based compounds. “No organism I know of would make these unique molecules.”
This article has been translated into Spanish by Divulgame.org and can be found here.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter