Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Low-cost chiral acid catalyst
Chiral Brønsted acid assembled in a few easy steps for about $5 per gram
by Bethany Halford
February 29, 2016
| A version of this story appeared in
Volume 94, Issue 9
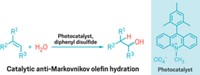
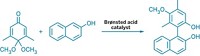
A common method for making one enantiomer of a small molecule is to use a chiral Brønsted acid to guide its synthesis. But enantioselective proton-donating acids can be expensive and laborious to make. Now, Columbia University chemists Tristan H. Lambert, Chirag D. Gheewala, and Bridget E. Collins have come up with a chiral Brønsted acid that can be assembled from inexpensive starting materials (Science 2016, DOI: 10.1126/science.aad0591). The catalyst (shown) is made by first reacting dimethyl malonate and dimethylacetylene dicarboxylate to create 1,2,3,4,5-pentacarbomethoxycyclopentadiene. This aromatic pentaester is then condensed with naturally occurring (–)-menthol. The chemists calculate that it costs about $4.00 per g to make the natural enantiomer of the catalyst and $5.50 per g to make the unnatural isomer. The catalyst’s aromatic stabilization is key to its proton-donating prowess. It works well in Mukaiyama-Mannich and oxocarbenium aldol reactions with catalyst loadings as low as 0.01 mol %. The Columbia team also used 1,2,3,4,5-pentacarbomethoxycyclopentadiene to prepare alternative chiral amide catalysts.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter