Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
ACS Meeting News
Remembering organic chemistry legend Robert Burns Woodward
Famed chemist would have been 100 this year
by Bethany Halford
April 10, 2017
| A version of this story appeared in
Volume 95, Issue 15

In brief
C&EN celebrates the 100th birthday of Robert Burns Woodward with this profile of the famous organic chemist. Read on as some of Woodward’s students and colleagues share their memories about his style and unique way of communicating chemistry. Also, chemists working in natural product synthesis reflect on what impact Woodward’s work has on the field today
Had Robert Burns Woodward lived to see his 100th birthday this week—on April 10—one wonders what the famed organic chemist would have thought of 2017. Renowned for his elegant and bold total syntheses of natural products, Woodward was also legendary for his collection of blue neckties, his chain-smoking, and his riveting three-hour lectures. But in an era where practitioners of total synthesis often face funding challenges, casual is the de rigueur dress code, smoking is verboten, and popular communication is frequently done in 140 characters or less, the Woodwardian way seems all but lost.
Even if Woodward is an anachronism in today’s world, it’s clear he had a profound impact on organic chemistry, particularly in the field of natural product synthesis. In celebration of Woodward’s centennial, C&EN wanted to introduce, or perhaps reintroduce, readers to this giant of 20th-century organic chemistry and explore how his work has influenced the practice today.
By modern metrics, Woodward’s tally of publications might strike some as modest: Woodward authored or coauthored 196 publications during his lifetime. But many organic chemists find Woodward in the branches of their academic family trees. By one estimate, he trained as many as 400 chemists—mostly postdocs and mostly men—over the course of his career.
“R. B. Woodward was a legend in his own time,” says K. C. Nicolaou, a synthetic organic chemist at Rice University who can trace his academic roots to Woodward. “Characterized by creativity, artistry, and elegance, his total synthesis endeavors represented a quantum jump in the molecular complexity that could be reached at the time.”
Woodward’s legacy remains strong. He died in 1979 at age 62, yet undergraduate chemistry students learn his name when they study the Woodward-Hoffmann rules, which he developed in collaboration with Cornell University’s Roald Hoffmann in the 1960s to describe the principles of molecular orbital symmetry that govern pericyclic reactions. Woodward’s syntheses of natural products date back more than 70 years, but they are still taught to graduate students in the field as a way to discuss strategy and risk taking.
“Woodward is, for the lack of a better word, an institution,” says Richmond Sarpong, a synthetic organic chemist at the University of California, Berkeley. “A test of his enduring nature is that his and [Harvard University synthetic organic chemist] E. J. Corey’s are the only names that most non-chemistry-major students in my sophomore organic chemistry course remember a year after they take the course.”
Woodward also has a grip on chemists’ imaginations beyond the chemistry he did. His masterful use of language has proved to be particularly captivating. A paper that chemistry historian and University of Richmond scholar Jeffrey I. Seeman published last year in Angewandte Chemie titled “Woodward’s Words” was one of the journal’s most accessed papers of 2016. “There are so many facets to Woodward that are extraordinarily appealing,” Seeman says.
A life in brief
Robert Burns Woodward was born in Boston on April 10, 1917. His father died the following year, and he was brought up by his mother, Margaret, in Quincy, just south of Boston.
Like many boys of his era, Woodward had a basement laboratory. As Woodward recalled when he received the American Chemical Society’s Arthur C. Cope Award (with Roald Hoffmann) in 1973, he had worked his way through most of the experiments in Ludwig Gattermann’s classic German laboratory manual, “The Practical Methods of Organic Chemistry.”
“I had found and purchased, in one of the delightful secondhand bookshops which then existed in Cornhill in old Boston, a copy of an English translation,” he told the attendees of the award reception.
Woodward was also reading intensively at this time. He walked more than 5 km to the public library to put his nose into any chemistry books he could get his hands on.
“I was only dimly aware, through allusions in those books, of the existence of the original journal literature,” he recalled. “I now decided that this matter deserved exploration and I took the forthright step of writing to the German consul-general at Boston—one Baron von Tippelskirch (really!)—intimating that I heard that chemical research was actively pursued in Germany, and that its fruits were described in publications which appeared at regular intervals. Could I enlist his kindness in helping me to procure samples of such publications?”
The baron was kind enough to oblige young Woodward, and among the journals he received was the 1928 issue of Liebigs Annalen der Chemie containing Otto Diels and Kurt Alder’s paper reporting what would come to be known as the Diels-Alder reaction—a transformation that captivated Woodward throughout his life.
In 1933, Woodward, aged 16, enrolled as an undergraduate at Massachusetts Institute of Technology. He “caused a good deal of consternation” at MIT, wrote his good friend and Oxford University chemist Alexander R. Todd in the Biographical Memoirs of Fellows of the Royal Society. “He quickly made it clear that he wanted to spend all his time as an undergraduate in the library and laboratory, to take final examinations without attending the set courses, and to forget about compulsory courses in physical education,” Todd notes. It nearly derailed Woodward. He was “excluded for inattention to formal studies” in 1934, according to his Nobel Prize biography, but he was allowed to reenroll the following year.
Woodward found an ally in MIT chemistry professor James Flack Norris. “We saw we had a person who possessed a very unusual mind and we wanted it to function at its best. If the red tape necessary for less brilliant minds had to be cut, we let it go,” Norris told the Boston Globe in June of 1937 when Woodward accomplished the dazzling feat of earning both his bachelor’s and doctoral degrees in a mere four years. “We think he will make a name for himself in the scientific world,” Norris added.
The following fall, Woodward took a position at Harvard University as an assistant to chemistry professor Elmer P. Kohler. He would stay there for the next 42 years, becoming a junior fellow and then moving up the faculty ranks to instructor, assistant professor, and so on, eventually holding several distinguished named chairs.
Woodward first made a splash in the chemistry community in 1941 and 1942 with his papers correlating ultraviolet spectra with structural elements, such as α, β-unsaturated ketones and conjugated dienes. What emerged from these papers were the Woodward rules (sometimes called the Woodward-Fieser rules because they were later corrected and expanded by Harvard chemists Louis and Mary Fieser), which empirically predict what wavelength a compound will absorb in the UV spectrum.
Chemists point to this as a trait that defined Woodward throughout his career: He embraced modern analytical techniques for advancing organic chemistry. First it was UV spectroscopy; later he would use infrared spectroscopy. He was also an early adopter of nuclear magnetic resonance and high-performance liquid chromatography.
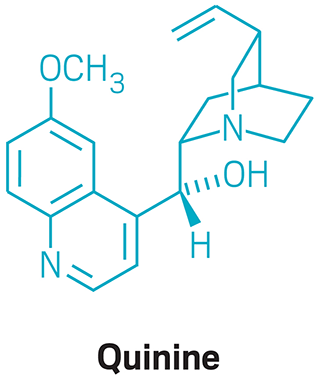
Woodward gained worldwide fame in 1944 when he completed a formal synthesis of quinine with William von Eggers Doering. This was during wartime, when the Allies had been cut off from their supply of this key malaria treatment. Headlines everywhere hailed the achievement. Life magazine ran beautiful photos of Woodward and Doering in the lab under the heading “Quinine: Two Young Chemists End a Century’s Search by Making Drug Synthetically from Coal Tar.”

Despite the acclaim, Woodward and Doering did not actually make quinine. Rather, they made quinotoxine, a compound that could be converted to quinine via a route devised by German chemists Paul Rabe and Karl Kindler in 1918. And Woodward and Doering made a scant 30 mg of quinotoxine. It was estimated that to produce quinine synthetically via their route would cost thousands of dollars per kilogram; it was simply too impractical to ever be implemented.
Even so, the beauty of Woodward and Doering’s synthetic strategy was undeniable, and it set Woodward on the path of natural product synthesis. Woodward also made great strides in natural product structure determination and, along with Geoffrey Wilkinson, he proposed the correct structure of ferrocene in 1952. But the total synthesis of natural products is where the lion’s share of hours in the Woodward lab went. It was “for his outstanding achievements in the art of organic synthesis” that Woodward won the 1965 Nobel Prize in Chemistry.
In 1963, the Swiss pharmaceutical company Ciba established the Woodward Research Institute in Basel and made Woodward its director. The institute boasted modern instrumentation and top-notch scientists dedicated to pursuing projects Woodward and Ciba envisioned.
The next year, Woodward began his collaboration with a young theorist named Roald Hoffmann, who was a junior fellow at Harvard but would soon take a faculty position at Cornell. Their work together would result in the now famous Woodward-Hoffmann rules.
Woodward’s work in total synthesis continued apace. He tackled larger and more complicated targets, notably vitamin B-12, a project he collaborated on with Albert Eschenmoser of the Swiss Federal Institute of Technology (ETH), Zurich. When Woodward died of a heart attack on July 8, 1979, in his Cambridge, Mass., apartment, his group was working on the total synthesis of the antibiotic erythromycin. The synthesis of the molecule was completed by his coworkers after his passing. But the Woodward Research Institute in Basel was shuttered.
Master strategist
To get an idea of just how revolutionary Woodward’s work was when he started constructing compounds in the 1940s, it’s important to understand how different his approach was from that of his chemical contemporaries. “When Woodward was a young chemist, people would make molecules from starting materials that kind of looked like the compounds that they wanted to make, and they would link them by known reactions,” says Erik Sorensen, a synthetic organic chemist at Princeton University. “But Woodward was known for his intellectual leaps that made many of his designs nonobvious. He was one of those very few chemists who changed the game by reasoning by mechanistic analogies.”
“He was a master at using relatively simple reagents—an acid here, a base there, a little bit of heat there—not particularly exotic reagents, but once masterfully arrayed, voilà!” adds Thomas Hoye, a synthetic organic chemist at the University of Minnesota, who was a graduate student in Woodward’s laboratory from 1972 to 1976.
Woodward’s work was less about inventing reactions, although he did do that on occasion. He didn’t, for example, design a reaction and then apply it over and over again to many natural products, a strategy that became common after Woodward’s death, says Thomas Maimone, a synthetic organic chemist at UC Berkeley. “Each target molecule instead represented a blank canvas and the unique peculiarities of the system helped dictate the overall strategy.”
Woodward’s synthesis of the once popular antihypertensive drug reserpine, published in 1956, is one that many chemists point to as a highlight in the history of total synthesis of natural products. “It was founded on visionary ideas and published right about the time organic chemists were worrying about how to utilize the principles of conformational analysis in synthesis design,” Sorensen says. Woodward built reserpine’s intricate structure using simple reagents. At the end of the synthesis, he and his group corrected an errant stereocenter by forcing an intermediate compound into an unfavorable conformation so that it epimerized into the correct stereoisomer. “Woodward’s insight into conformational preferences to solve that problem is really quite striking,” Sorensen notes.
Sorensen points to Woodward’s synthesis of chlorophyll, which took four years to complete and was published in 1960, as a prime example of strategic risk taking in organic synthesis. Woodward thought the molecular architecture that defines the chlorins—a structural family that includes chlorophyll—might originate from a highly substituted porphyrin with a crowded periphery because of structural similarities, Sorensen explains.
Advertisement
So he convinced his students and postdocs that they should synthesize this type of molecule in an attempt to eventually reach chlorophyll. “Woodward reasoned that by building such a system that they would engineer into that porphyrin a strong driving force that might allow his students to directly convert it into a chlorin,” Sorensen says.
The setup costs for constructing this crowded porphyrin, in terms of time and labor, were high, Sorensen points out. And Woodward didn’t really know if the final conversion to a chlorin would work.
It did. Just by heating the porphyrin in acetic acid, the team got the molecule to isomerize as Woodward had predicted. “I still think this is one of the boldest, most daring designs of all time in the field of organic natural product synthesis,” Sorensen says.
“You can’t overstate the impact that Woodward had on the field of natural product synthesis,” says Sarah Reisman, a synthetic organic chemist at California Institute of Technology. Reisman enjoys teaching how a problem Woodward and his team encountered during the total synthesis of vitamin B-12 led to the Woodward-Hoffmann rules.
“It’s a beautiful example of how an observation from a synthetic effort played a fundamental role in teaching us something very important about chemistry,” she says. “It’s undeniable how important and how powerfully predictive the Woodward-Hoffmann rules have turned out to be for chemists. I love that connection.”
“To me, Woodward’s contribution to natural product synthesis comes in large measure from the way his amazing accomplishments inspired chemists,” says Chris Vanderwal, a synthetic organic chemist at the University of California, Irvine. “However, some of his stunningly creative approaches were more difficult to learn from because he wouldn’t always let us less-creative types in on his thought processes.” Vanderwal cites Woodward’s prostaglandin and cephalosporin syntheses as cases “where the provenance of the design is far from obvious.”
Sorensen agrees. “When I teach Woodward, it’s hard to take the final product and systematically reduce its complexity for students because his syntheses tend to be replete with these little jumps that are sort of unique to Woodward,” he says. “They don’t give you a road map that you could use to solve other problems.”
Preeminent problem solver
Even those who studied with Woodward say it was tough to gain insight into how he dissected problems. Graduate students say they spent little time with him one-on-one, and when they did, he revealed little about what he was thinking as he would work his way through a problem or devise a strategy.
“It was hard to get Woodward’s time. He was busy,” says Hoffmann, Cornell University chemistry professor and Woodward’s collaborator on the famous Woodward-Hoffmann rules of orbital symmetry. “But when you had his time, you had this feeling that all the intellectual power of the world was focused on what you were telling him. There was undivided attention. Complete understanding. Not too many words. But you got the feeling of cogitation—of a person thinking.”
Woodward’s intellect was often on display at his famous Thursday-night seminars, which would start at 8 PM and last into the wee hours of Friday morning. “Here many brilliant ideas would emerge, apparently spontaneous and unpremeditated,” wrote Todd in his biographical memoir of Woodward, “and some at least of his audience got the impression that all that was necessary to produce brilliant ideas was a bottle of whisky and several packs of cigarettes.”
David M. Lemal, a Dartmouth College chemistry professor, was a graduate student in Woodward’s group from 1955 to 1958. He recalls one particular Thursday-night seminar when another graduate student who seemed to have no good prospect of getting a degree anytime soon presented a large body of work done by German chemist Otto Diels in the early 1900s. It was just a large number of different reactions, as Lemal remembers, and it was clear, with the gains chemistry had made in the intervening decades, that Diels’s interpretation of the chemistry was incorrect.
After the student’s presentation, Woodward asked everyone in the room to try to figure out what was actually going on. After 90 minutes of silence and furious scribbling, “Woodward got up and went to the blackboard and reinterpreted this body of work from beginning to end with great clarity in his usual blackboard work that you can photograph and put in a textbook,” Lemal says. Woodward sent the graduate student into the lab to repeat the work with modern analytical tools. The student was able to confirm Woodward’s conclusions and in short order wrote his Ph.D. thesis and graduated.
“What he did so well was to go on thinking about a problem much longer than anybody else would have done,” says Ian Fleming, an organic chemist at the University of Cambridge, who was a postdoc with Woodward from 1963 to 1964. “He showed that by thinking hard about a problem you could get much further than you realized.”
Fleming recalls his arrival at Harvard in 1963 with fellow postdoc Jean-Marie Lehn, who is now an organic chemistry professor at the University of Strasbourg. The two young chemists met Woodward in his office and were given a five-hour lecture about the vitamin B-12 synthesis project they would be joining. Woodward spoke about everything that had been done up to that point—a point at which the team had reached an impasse.
“He was doing it to clear his own mind, quite obviously. And Jean-Marie and I were the only ones who were given this treat,” Fleming recalls. “He’d gone through about 10 cigarette packages by the end of the day.”
Fleming says he was lucky to get to speak with Woodward frequently about chemistry during his time as a postdoc. “There were others in the group who got nothing because what they were doing wasn’t leading quickly to anything for him to think about and so he wasn’t that interested. He was like that. He was ruthless with following the people he decided would give him something to think about.”
Many chemists note that the actual chemistry Woodward did has largely fallen out of favor. “Woodward’s influence on organic chemistry today is on the one hand small, as would be expected for a field whose capabilities are growing exponentially,” says Stuart L. Schreiber, a chemical biologist at Harvard University and the Broad Institute, who was the last student to earn a doctoral degree in Woodward’s lab. “On the other hand, I think the intrinsic beauty of organic chemistry that excites many young students today can be traced to the role Woodward played in illuminating the art in organic chemistry. Woodward still has a spiritual presence in our field, even though we are not heavily dependent on the tools and insights he made available decades earlier.”
A colorful personality
Stepping into the chemistry library at Harvard University—with its crimson carpet, wood-paneled walls, glowing chandeliers, and second-floor balconies—it almost seems possible to travel back to a time when graduate students could catch glimpses of Woodward sifting through the chemical literature, probably puffing on a Benson & Hedges cigarette—his preferred brand.
Dudley Herschbach, a chemistry professor at Harvard, recalls a time in the mid-1970s when he was the chemistry department’s chair and had to negotiate a détente between Woodward and chemistry professor William Lipscomb, who had emphysema, over Woodward’s smoking in the library. “Woodward, I have to say, was very gracious,” Herschbach recalls. He didn’t want a rigorous rule but instead drafted a typical Woodwardian phrase about graciously not smoking that Lipscomb’s wife, who was a skilled calligrapher, wrote out on signs posted throughout the library in blue ink.
Woodward’s legendary love of blue cannot go unmentioned. He wore blue suits and blue ties, drove blue cars, and his students painted a rectangle of blue to cover his parking space at Harvard.
His lectures, which were invariably titled “Recent advances in the chemistry of natural products” and rarely lasted less than three hours, were legendary as well. In his Cope Award address, Woodward credited University of Basel theoretical chemist Edgar Heilbronner with establishing the “Woodward” as a unit of lecture time lasting five hours and 20 minutes.
“Bob’s megalectures were not displays of arrogance—or anyway not primarily so,” wrote his fellow Harvard chemistry professor Frank Westheimer in the 2001 book “Robert Burns Woodward: Architect and Artist in the World of Molecules.” They were based, Westheimer noted, on Woodward’s drive for perfection. “If a subject required three hours to explain properly, then he would give it three hours and expect his audience to do likewise.”
“Just his words demonstrate sophistication and beauty,” remarks the University of Richmond’s Seeman, who has devoted much of the past decade to studying Woodward’s writings and personal correspondence. “It’s like listening to a symphony orchestra when you read some of his papers or letters.”
Advertisement
One of Seeman’s favorite stories about Woodward involves a letter he wrote to a high school student named Allen Hoos in 1961. Hoos had written to Woodward to ask why, since he was planning to become a chemist, he had to take “so much English.” Hoos queried Woodward: “Is English that important in the field of chemistry?”
“It is a very valuable asset to a chemist to be able to formulate his ideas, describe his experiments, and express his conclusions in clear, forceful English,” Woodward wrote back to Hoos. “Further, since thought necessarily involves the use of words, thinking is more powerful, and its conclusions are more valid, in the degree to which the thinker has a command of language.”
Seeman says he finds Woodward’s response touching, “not solely because of the thoughtfulness of the response and not solely because of the beauty of the way he writes, but because of the fact that he would take the time to single out this one letter. There was a generosity of spirit and scholarship and culture that Woodward wanted to share.”
At the same time, Woodward was not without his flaws. Elitist, prickly, arrogant—these are a few of the adjectives those who knew Woodward use to describe him when pressed. Harvard’s Corey has said that he had a conversation with Woodward in 1964 that provided the basis for what would become the Woodward-Hoffmann rules but received no acknowledgment or credit.
But as the Latin proverb goes, de mortuis nihil nisi bonum (of the dead, say nothing but good). One hundred years from now, Woodward will not be remembered for his shortcomings but for putting art into chemistry. So that’s the subject on which we will give him the last word, quoted from his Cope Award address. This is his explanation for choosing to study chemistry instead of mathematics—his attraction to the field’s more palpable aspects:
“I love crystals, the beauty of their form—and their formation; liquids, dormant, distilling, sloshing!; the fumes; the odors—good and bad; the rainbow of colors; the gleaming vessels, of every size, shape, and purpose. Much as I might think about chemistry, it would not exist for me without these physical, visual, tangible, sensuous things.”
Woodward through the years
Woodward through the years
April 10, 1917
A modest beginning
Robert Burns Woodward is born in Boston.
1937
The young doctorate
Woodward graduates from MIT with a Ph.D. in chemistry at the ripe old age of 20.

1944
En route to quinine
Woodward and William von Eggers Doering make headlines when they publish what today would be called a formal synthesis of the antimalarial drug quinine.

1950
Rising in the faculty ranks
Woodward becomes a full professor at Harvard.

1951
Biomolecule syntheses
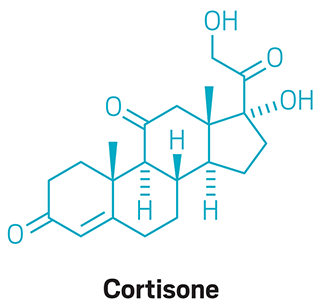
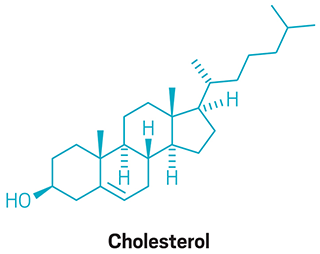
Cholesterol and Cortisone are crafted in Woodward’s laboratory.
1952
Organometallic origins
Along with Geoffrey Wilkinson, Woodward suggests the correct structure for ferrocene.

1954
Alkaloids ahoy!
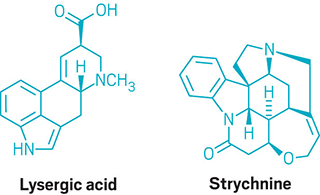
Woodward’s group tackles the syntheses of lysergic acid and strychnine, to name just a few of the naturally occurring alkaloids the researchers made
1956
One of the classics
Chemists often point to Woodward’s synthesis of the once popular antihypertensive drug reserpine as one of his most creative and artistic.
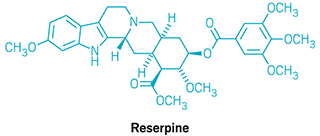
1960
Green chemistry
After four years, the Woodward group succeeds in synthesizing chlorophyll.
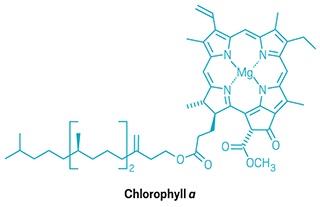
1964-69
The Woodward-Hoffmann rules
Woodward and theorist Roald Hoffmann collaborate on what will become the famous rules of orbital symmetry. In 1981 Hoffmann shares the Nobel Prize in Chemistry for this work with Japanese chemist Kenichi Fukui, who had developed a similar theroy independently. Scholars agree that Woodward would have shared this prize had he not passed away.

1965
The Nobel nod
Woodward wins the Nobel Prize in Chemistry :for his outstanding achievements in the art of organic synthesis."
1965-1966
Complex targets
The Woodward group publishes total syntheses of the gout treament colchicine and the antibiotic cephalosporin C.
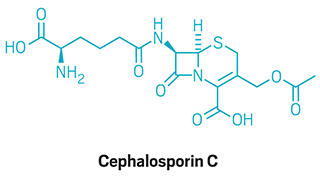
1976
A vitamin to be reckoned with
After working for many years and teaming with nearly 100 group members, Woodward and ETH Zurich’s Albert Eschenmoser succeed in synthesizing vitamin B12.
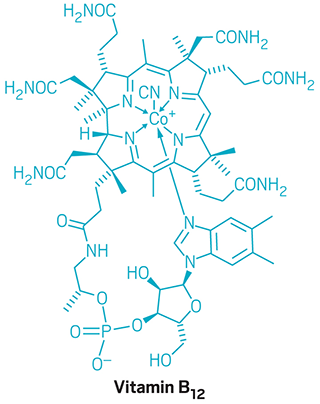
July 8, 1979
An unexpected passing
Woodward dies at age 62.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter