Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Policy
Sunscreen approval delays prompt push for high-throughput tests
by Britt E. Erickson
January 29, 2018
| A version of this story appeared in
Volume 96, Issue 5
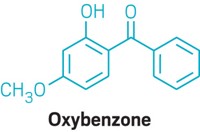
Sunscreen manufacturers are urging the U.S. FDA to relax testing requirements for new active ingredients, claiming the tests are too costly, animal intensive, and slow. Companies want instead to use high-throughput, nonanimal methods. Animal welfare groups and some members of Congress support the idea. In a Jan. 17 letter to the director of the National Toxicology Program Interagency Center for the Evaluation of Alternative Toxicological Methods, Rep. Robert Pittenger (R-N.C.) asked what the center is doing “to facilitate the regulatory acceptance of alternatives to animal testing for sunscreen ingredients.” The Sunscreen Innovation Act, enacted in 2014, set deadlines for FDA to review new sunscreen active ingredients, but it did not eliminate the need for FDA to determine that each substance is “generally recognized as safe and effective.” Eight ingredients—amiloxate, bemotrizinol, bisoctrizole, drometrizole trisiloxane, ecamsule, enzacamene, iscotrizinol, and octyl triazone—were under review when the law was enacted, and they still are. FDA requested additional toxicity data for all of them, but manufacturers have yet to comply. The deadline clock starts after FDA receives the data.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter