Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biochemistry
Phosphorylation at different sites of cancer-related enzyme leads to different shape changes
Recent work might have implications for drug development
by Celia Henry Arnaud
August 2, 2018
| A version of this story appeared in
Volume 96, Issue 32
Through its job of adding phosphate groups to target proteins, the kinase Akt is involved in regulating cell proliferation (and, therefore, cancer), glucose metabolism, and transcription, among many other functions.
Though researchers worked out some details governing Akt activation in the 1990s, they still don’t have all the pieces of the puzzle, which makes targeting Akt with drugs all the more challenging.
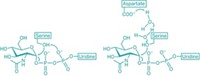
For instance, scientists know that phosphorylation at serine-473 activates Akt, and that phosphorylation at serine-477 and threonine-479 also turn the enzyme on. But researchers haven’t known if those options lead to the same or different activation states.
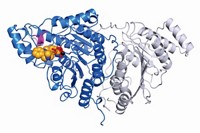
Philip A. Cole of Harvard Medical School, Sandra B. Gabelli of Johns Hopkins School of Medicine, and coworkers now use chemical methods to make site-specifically phosphorylated versions of Akt (Cell 2018, DOI: 10.1016/j.cell.2018.07.003). They use the various forms for biochemical, crystallographic, and cellular analyses of the kinase’s activation mechanisms.
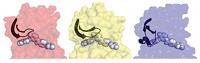
Although serine-473 phosphorylation and dual serine-477/threonine-479 phosphorylation both lead to active forms of the enzyme, the two forms act upon their substrates with different catalytic efficiencies. They also appear to undergo different shape changes on their way to becoming active.
Researchers previously thought that phosphorylation at serine-473 works by interacting with and stabilizing the kinase domain. The new study shows that the phosphate interacts with the linker between the kinase domain and another part of the protein called the PH domain. Researchers thought the linker was unimportant, Cole says, but this work suggests that it’s actually quite important.
Akt has three known forms, and the linker is different in each, says Alex Toker, an Akt expert at Harvard Medical School who wasn’t involved in the study. The fact that the phosphate might interact with these different linkers during activation could account for different functions ascribed to the different Akt forms, particularly in cancer.
In contrast, serine-477/threonine-479 phosphorylation works by displacing the PH domain and reducing the enzyme’s ability to turn itself off. But the current research doesn’t nail down how the phosphorylation actually accomplishes the displacement, Cole says.
“It’s quite possible, but not yet proven, that these different activation states of Akt have different biological roles,” Cole says. In biochemical studies, the forms responded differently to small-molecule kinase inhibitors. Thus it’s important for drug developers to be cognizant of the two states, Cole says.
In the future, Cole hopes to work with drug developers to identify inhibitors that target specific Akt activation states.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter