Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biochemistry
Covid-19
Researchers in China report structure of the novel coronavirus bound to its human target
The structure shows the first steps of SARS-CoV-2 infection, and could help in drug discovery
by Megha Satyanarayana
March 6, 2020

In another step toward developing treatments and vaccines against the novel coronavirus, SARS-CoV-2, a team of researchers in China have reported the crystal structure of a part of the virus bound to its target on human cells (Science, 2020. DOI: 10.1126/science.abb2762.
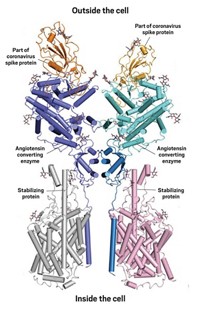
The snapshot of this interaction, captured through cryogenic electron microscopy by Qiang Zhou of the Westlake Institute for Advanced Study and colleagues, reveals some of the chemistry behind how the coronavirus hijacks angiotensin converting enzyme (ACE2), an enzyme involved in blood pressure regulation. The researchers think that the structure could lead to the development of antibodies that block this interaction.
The new cryo-EM structure comes hot on the heels of another revealed in February that showed the full viral spike protein, which is the part of the virus that binds ACE2 (Science, 2020. DOI: 10.1126/science.abb2507). Jason McLellan, the University of Texas at Austin researcher who led the team behind the spike protein structure, says that Zhou’s team’s work will help scientists better understand how coronaviruses have evolved to use this critical enzyme to get into human cells. He also agrees that the interaction captured in the structure could inspire the development of neutralizing antibodies. McLellan’s work with the coronavirus spike protein is being developed into a vaccine.
ACE2 is the first in a string of enzymes that convert the hormone angiotensin into its active form. When cleaved by enzymes, angiotensin makes blood vessels contract. The SARS-CoV-2 spike protein has two key elements involved in infecting human cells. A string of amino acids in the S1 subunit directly binds to the protein-cleaving part of ACE2 called the peptidase domain. The S2 subunit of the spike protein helps the virus fuse to the human cell. The new structure shows the first of these two events.
Support nonprofit science journalism
C&EN has made this story and all of its coverage of the coronavirus epidemic freely available during the outbreak to keep the public informed. To support us:
Donate Join Subscribe
“You have attachment, and entry. Blocking either function can prevent entry,” McLellan says, describing how treatments could be designed to stop SARS-CoV-2. “Ideally, you want antibodies that can target both functions.”
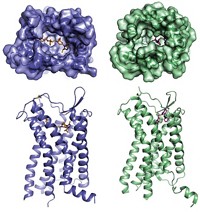
The ACE2 protein has a section that winds through the cell membrane, and that section has been difficult to crystallize. To overcome this, Zhou and his team paired it with another protein, an amino acid transporter, that it interacts with in cells. Once the researchers crystallized the ACE2 complex, they added a portion of the spike protein’s S1 subunit called the receptor binding domain. It’s not clear if that amino acid transporter plays any role in coronavirus infection.
The scientists found that the protein-cleaving part of ACE2 binds the spike through polar interactions formed from a bridge-like structure on the enzyme. Both ends of the receptor binding domain stick to ACE2 through hydrogen bonding and van der Waals forces, and in the middle, Zhou describes several amino acids that interact with an asparagine and histidine in ACE2 that may be required for the spike protein-ACE2 interaction to occur.
McLellan says that SARS-CoV-2 binds ACE2 more strongly than does the virus that caused the severe acute respiratory syndrome outbreak in 2003. Zhou’s research shows the subtle amino acid changes that create salt bridges and improve van der Waals interactions that might underlie this stronger interaction, he says.
Efforts to repurpose ACE2 inhibitors to block coronavirus infection have not been successful in the past, and McLellan says that given what has been revealed in this structure, it would be hard to develop a small molecule inhibitor that could squeeze between the virus and ACE2. Neutralizing antibodies, he says, might be a better bet.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter