Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Neuroscience
Is Parkinson’s disease caused by dysfunctional lipids?
As scientists think beyond α-synuclein, multiple pathways in lipid metabolism provide targets for potential drugs
by Celia Henry Arnaud
July 18, 2021
| A version of this story appeared in
Volume 99, Issue 26

James Parkinson, a 19th-century British surgeon, first reported what he called “the shaking palsy” in 1817, after observing a handful of patients with tremors and difficulty moving. More than 200 years later, the cause of Parkinson’s, the neurodegenerative disease that now bears his name, remains unknown.
For decades, a protein called α-synuclein has been the lead suspect as the culprit in Parkinson’s, which is marked by the loss of dopamine-producing neurons and motor dysfunction. When studying the brain tissue of people who have died of the disease, pathologists find dense clumps in the brain, especially in dopamine-producing neurons. These clumps, known as Lewy bodies, are packed with α-synuclein that is misfolded and aggregated.
If the phenomenon sounds familiar, that’s because protein clumps are a hallmark of another common neurodegenerative disease, Alzheimer’s disease. Researchers have spent decades and billions of dollars developing drugs that can prevent or break up plaques made of the peptide amyloid-β, hypothesizing the drugs would stem the neurological decline in people with Alzheimer’s. But so far, despite the recent approval in the US of an amyloid-targeting drug, the evidence that the approach slows the disease is limited. It’s still unclear whether the plaques are a cause or an effect of Alzheimer’s.
Similarly, the role of α-synuclein and Lewy bodies in Parkinson’s remains unclear, and the research community sees the amyloid story as a cautionary tale against relying on a single target.
“The evidence that synuclein plays a direct role in Parkinson’s is extremely weak,” says Peter T. Lansbury Jr., chief scientific officer of Bial Biotech and a professor of neurology at Harvard Medical School. “Without the amyloid story, it would be so weak that it would be just 1 of 15 ideas out there.” Lansbury was an early champion of a causative role for α-synuclein. Now he’s not so sure.
Higher levels of α-synuclein are associated with increased risk of Parkinson’s disease. “In that sense, it is probably the major player, but it is not the only player,” says Sreeganga Chandra, who studies the biology of synapses at Yale University.
Identifying the role of Lewy bodies has been a challenge, Chandra says, because they were first discovered after people had died. Even today, by the time people are diagnosed with Parkinson’s, their disease is advanced. That’s because early symptoms, such as constipation and sleep disruption, are common to other disorders and can precede the development of motor dysfunction by as much as 20 years. The Lewy bodies are usually studied after they are fully formed, and trying to retrace how they formed is difficult, she says.
And while large-scale genomic studies have identified rare genetic variants that increase the risk of Parkinson’s, only some of those variants are of the gene for α-synuclein, adding to skepticism that the protein is working alone to cause the disease.
Many Parkinson’s-associated variants are in enzymes involved in different parts of pathways used to build and break down lipids. And researchers have found that when the balance of lipids changes, “α-synuclein starts accumulating in toxic forms,” says Ronit Sharon, a neuroscientist at the Hebrew University of Jerusalem who studies Parkinson’s-associated changes in lipid composition in the brain. Conversely, when α-synuclein mutations are introduced into animal models, the mix of lipids changes. “It goes together. It’s very difficult to say which comes first.”
An increasing number of scientists are wondering: Could Parkinson’s be, at least in part, a disease of lipid metabolism? Some of them are starting to more closely explore lipids’ role in the development of the disease—and even develop drugs for Parkinson’s that modulate lipid metabolism.
Getting the biochemical picture
The lipid connection comes as no surprise to people who study α-synuclein. The protein’s normal function is poorly understood, but researchers know a bit about where and with what it interacts. “It’s clearly a membrane-binding protein,” says David Eliezer, who studies α-synuclein at Weill Cornell Medicine. The protein binds to synaptic vesicles, tiny lipid containers in neurons that are used to package neurotransmitters. It seems to influence how the containers move in and out of cells, how they are stored, and how big they get, Eliezer says. “α-Synuclein’s fundamental biology is probably strongly linked to lipid biology,” he adds.
The protein is drawn to membranes that feature negatively charged head groups and have the right shape and fluidity, says Ulf Dettmer, whose lab studies α-synuclein biology at Brigham and Women’s Hospital and Harvard Medical School. “It turns out that synaptic vesicles have the right composition and right curvature for synuclein to bind,” he says. As a research fellow in Dennis Selkoe’s lab, also at Brigham and Women’s Hospital and Harvard Medical School, Dettmer designed α-synuclein variants that exhibit increased membrane binding. These variants cause Parkinson’s-like symptoms in mice (Neuron 2018, DOI: 10.1016/j.neuron.2018.09.014). Dettmer’s lab is using the variants to better understand how synuclein-related pathologies develop and how they could be treated.

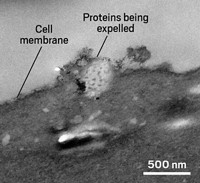
Structural studies of Lewy bodies underscore the connection to lipids. Wilma D. J. van de Berg of Amsterdam UMC at the VU University Medical Center and her colleagues obtained high-resolution structures that are changing the field’s understanding of Lewy bodies. The team pinpointed Lewy bodies in human brain tissue using α-synuclein as a marker and then visualized them using a combination of light microscopy and electron microscopy (Nat. Neurosci. 2019, DOI: 10.1038/s41593-019-0423-2). The structures were a complex mixture of organelles and lipid membranes. In analyses of lipid composition, the researchers found that sphingomyelin is highly enriched in Lewy bodies.
In subsequent work, van de Berg and her colleagues have shown how the proteins and lipids in Lewy bodies are arranged. Phosphorylated α-synuclein, intact mitochondria, and cytoskeletal proteins called neurofilaments form a framework that encloses damaged membranes and organelles in Lewy bodies (Acta Neuropathol. 2021, DOI: 10.1007/s00401-021-02329-9).
Van de Berg thinks proteins and lipids don’t just passively clump together. “We see that the Lewy body is highly organized and very well structured,” she says. There’s even discussion in the field, Lansbury says, that Lewy bodies might be protective.
The structural work may provide clues to how Lewy bodies assemble. Van de Berg has found smaller aggregates of different sizes and shapes that also contain α-synuclein. “I think the smaller aggregates can tell us a lot about how these are formed and how they’re different among neurons, maybe even among patients,” she says.
“The bottom line is most of the pathology in Parkinson’s disease is membrane pathology,” says Robert Edwards, a neuroscientist at the University of California, San Francisco, who studies the interactions of α-synuclein with membranes. “That’s why membrane interactions are going to be really important, regardless of whether the [α-synuclein] aggregation is extreme.” He thinks α-synuclein aggregation is a downstream result of the disease rather than its cause.
Some people are trying to determine how the interplay between α-synuclein and lipids leads to the formation of these Lewy bodies and how things go wrong. “We know that there are lipid changes,” says Saranna Fanning, a neuroscientist in Selkoe’s lab at Brigham and Women’s Hospital and Harvard Medical School. Measurements of plasma and cerebrospinal fluid have shown alterations in lipids in response to excess or mutated α-synuclein. “It’s a little hard to dissect because looking at a postmortem brain is not necessarily the most informative in terms of what’s happening in the early biology,” Fanning says.
Chandra and her colleagues have shown that certain classes of lipids have different effects on α-synuclein aggregates, influencing how fast the proteins come together or how large they become (J. Neurosci. 2017, DOI: 10.1523/JNEUROSCI.1525-17.2017). “Glucosylsphingosine and sphingosine make very defined, small-size, oligomeric species,” Chandra says. “If you add a glucosylceramide, then you make much longer species, almost fibrillar.” She doesn’t yet have detailed images that can explain how or even if the lipids are incorporated in the aggregates. “But clearly there’s something that caps the reaction,” she says. She is studying whether tweaking the levels of acid ceramidase, an enzyme that uses glucosylceramide to make glucosylsphingosine, affects oligomer and fibril formation in stem cell neurons.
Fanning, Dettmer, and their colleagues have found that the amount of neutral lipids such as di- and triglycerides increases in the presence of excess or mutated α-synuclein (Molec. Cell 2018, DOI: 10.1016/j.molcel.2018.11.028). “We know that you get an accumulation of lipid droplets storing excess fatty acid that could otherwise be cytotoxic,” Fanning says. “We think monounsaturated fatty acids, such as oleic acid and palmitoleic acid, are particularly important in the biochemistry of Parkinson’s disease.”
Sharon hopes to find changes in the biochemistry of the brain that allow earlier diagnosis, and ultimately treatment, of Parkinson’s. “I believe it is all about the lipid in Parkinson’s,” she says. Identifying the early changes in lipids could pave the way for developing drugs that modify the course of the disease rather than simply treating symptoms.
Real-world studies
Several enzymes involved in lipid metabolism, either directly or as regulators, are being targeted for potential Parkinson’s treatments.
For example, genetic studies have shown that mutations in the gene GBA1, which encodes the enzyme glucocerebrosidase, and in LRRK2, which encodes the enzyme leucine-rich repeat kinase, also called LRRK2, elevate the risk of developing Parkinson’s. Both genes are associated with lysosomes. These organelles contain enzymes that digest damaged biomolecules, including certain classes of lipids, into building blocks that can be recycled. But if one or more of those enzymes don’t work properly, damaged molecules accumulate, and new molecules can’t be made.
Both glucocerebrosidase and LRRK2 are also associated with the movement of membrane materials. Disrupted membrane trafficking could contribute to lipid aggregation.
Drugmakers are taking note. Bial Biotech is developing a Parkinson’s drug that targets glucocerebrosidase, which breaks glucocerebroside into glucose and ceramide. The ceramide is then involved in the synthesis of sphingolipids. Mutations in glucocerebrosidase that reduce its ability to process glucocerebroside are associated with an increased risk of Parkinson’s. Other reactions involved in sphingolipid recycling also increase the risk of Parkinson’s.

“My hypothesis is that anything that changes the rate of flux through [the sphingolipid recycling] pathway is bad,” Lansbury says. “The turnover of these sphingolipids determines the properties of all your cell membranes.” Slow sphingolipid turnover makes membranes less adaptable and responsive; rapid turnover makes membranes very adaptable, he says.
Bial’s drug candidate has completed short-term dosing trials, and the company expects to start a yearlong trial in 300–400 people with Parkinson’s in July 2022.
Denali Therapeutics is developing compounds that target LRRK2. “From a genetic perspective, LRRK2 is really compelling,” says Anastasia Henry, associate director at Denali. “There are variants that are associated with increased risk for Parkinson’s disease but also variants that are associated with reduced risk, suggesting it can be protective in some context.”
Researchers made the connection between Parkinson’s and LRRK2 before they knew what molecule the enzyme acted on. Then in 2016, a team of scientists showed that LRRK2 phosphorylates members of a family of enzymes called Rab GTPases, which are involved in regulating membrane trafficking (eLife 2016, DOI: 10.7554/eLife.12813). “That was actually a huge game changer for the field,” Henry says. It also allowed Denali to identify biomarkers for its LRRK2 inhibitors. “We can look at phosphorylation of these Rabs in the clinic to really understand if we’re hitting the target.”
Denali’s LRRK2-targeting compound has proved its safety in volunteers without Parkinson’s and has completed an early-stage study in people with Parkinson’s. The biotech firm is using bis(monoacylglycero)phosphate (BMP) as a biomarker. When LRRK2 activity decreases, the amount of BMP also decreases. Henry says the company is mapping out the late-stage development of the drug, which it plans to study both in people who have LRRK2 variants known to increase the risk of Parkinson’s and in a broader population of people with Parkinson’s.
Companies are also chasing a third enzyme, stearoyl-CoA desaturase (SCD), which does not have a known genetic link to Parkinson’s.
This enzyme removes hydrogens from fatty acids with C16 or C18 chains. This removal introduces a double bond, which changes the shape of the fatty acids as well as the properties of membranes made with them. The hypothesis is that α-synuclein doesn’t bind as well to membranes with higher levels of unsaturated lipids. SCD was identified as a potential Parkinson’s target by screening yeast cells for genes that counteract the effects of large amounts of α-synuclein (Cell Rep. 2018, DOI: 10.1016/j.celrep.2018.11.028). Studies in mice suggest that blocking SCD can ameliorate the effects of excess α-synuclein.
Advertisement
The biotech firm Yumanity Therapeutics has an SCD inhibitor in human trials. Blocking the enzyme should decrease the amount of unsaturated fatty acids and increase the amount of saturated fatty acids. To monitor the effectiveness of its drug candidate, the company measures the ratio of the two fatty acids, a biomarker known as the fatty acid desaturation index.
“The beauty of that is that we can measure it in the plasma,” says Ajay Verma, executive vice president and head of research and development at Yumanity. The company thinks SCD needs to be inhibited by only about 20% to be effective. The company has completed a safety study in 72 volunteers without Parkinson’s and is conducting a small trial in people who have Parkinson’s.
These clinical studies might provide some answers about the lipids’ role in the progression of Parkinson’s. The combination of real-world studies and ongoing basic science could clarify the roles of all the players, lipids and α-synuclein alike. Identifying what α-synuclein normally does will help with that.
“We’re just trying to understand the normal function of the protein,” UCSF’s Edwards says. “Then all of these things—its interaction with lipids, its interaction with other proteins—will fall into place.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter