Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Consumer Products
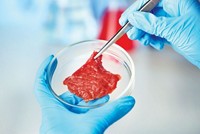
FDA, USDA to share oversight for cell-based meat
by Melody M. Bomgardner
November 20, 2018
| A version of this story appeared in
Volume 96, Issue 47

Less than two months after holding a joint public meeting about safety and marketing of foods made from cultured meat cells, the U.S. Food & Drug Administration and the Department of Agriculture have agreed to share regulatory responsibility for the products. Several start-ups, including Memphis Meats and Mosa Meat, plan to commercialize poultry- and beef-like products made from live cells grown in bioreactors. But until now it was not clear how the production processes or the products would be regulated. The agreement states FDA will oversee cell collection, cell banks, and cell growth and differentiation processes. Responsibility will transition to USDA during the cell harvest stage. USDA will then have oversight over production and labeling of the food products. Both agencies say that no additional legislation will be necessary. The Good Food Institute, a proponent of the cell-based meat industry, says timely rules will help the U.S. keep pace with advances in Israel, Japan, and Singapore. “This announcement is an exciting indication that FDA and USDA are clearing the way for a transparent and predictable regulatory path forward,” says Jessica Almy, GFI’s director of policy.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter