Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Energy
Reactions: Separating gasoline from crude oil, and using thermodynamics to answer hydrogen peroxide question
July 23, 2022
| A version of this story appeared in
Volume 100, Issue 26
Letters to the editor
Separation membranes
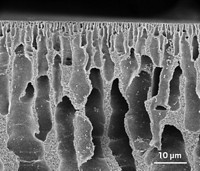
As a scientist and engineer working on petroleum research and production, I am happy to see the prototype membrane system for the direct separation of gasoline from crude oil, as reported in C&EN (June 20, 2022, page 4) and Science (2022, DOI: 10.1126/science.abm7686). While nowadays most petroleum products, such as gasoline or diesel, are refined from crude in oil refinery plants, alternative and novel approaches such as the above-mentioned membrane method should be encouraged, endorsed, and enhanced. Dealing with various crude oil samples on a routine basis, I feel that one of the key areas for improvement is the mitigation of pore blocking in the membrane, which is also hinted in the team’s Science paper. Crude oil is a liquid mixture of mainly hydrocarbons with a spectrum of molecular weights, and the high-molecular-weight components like asphaltenes and waxes can easily precipitate out and block the micro- and nanopores in the membrane, quickly disrupting its filtration function. I notice that the crude samples used in the team’s research were light crude oil (API gravity > 31.1°), which typically has much lower levels of asphaltenes or waxes than heavy crude but may still accumulate asphaltenes and waxes over time into membrane pores to eventually block the filtration process. In practical operations, this means the replacement or rejuvenation of the membrane filters, which adds to the operating cost. If heavy crude oil is involved, the cost is expected to go further up. The new membrane method also faces the scale-up challenge in order to compete on the cost-effectiveness with current oil refinery facilities that can process over 120,000 m3 of crude oil every day.
Leiming Li
Sugar Land, Texas
Thermodynamics in hydrogen peroxide reaction
The question of whether water can form hydrogen peroxide should be answered by thermodynamics (see C&EN, June 13, 2022, page 3). If a reaction is possible (spontaneous), then the change in the Gibbs energy at constant temperature and pressure must be negative in sign. For the reaction of water with air (O2) to form hydrogen peroxide, the change in the Gibbs energy at standard temperature and pressure is positive (+116.78 kJ/mol for liquids or +136.25 kJ/mol for gases). Therefore, the production of hydrogen peroxide by reactions of water with oxygen is not possible for such laboratory conditions.
Thermodynamics never depends on any proposed reaction mechanisms, such as sprayed water droplets or the condensation of such water vapor on inert substrates.
Melvin H. Miles
St. George, Utah


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter