Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Energy Storage
Porous organic crystals raise hopes for hydrogen storage
Molecules assemble into interlocked networks that can pack in plenty of hydrogen, albeit at low temperatures
by Mark Peplow, special to C&EN
September 5, 2024
| A version of this story appeared in
Volume 102, Issue 28

A robust crystal made from organic molecules can squeeze copious amounts of hydrogen into its pores, offering a promising way to store the gas (Nat. Chem. 2024, DOI: 10.1038/s41557-024-01622-w). The crystal is a hydrogen-bonded organic framework (HOF), and it ranks among the best hydrogen storage materials discovered to date, says J. Fraser Stoddart of the University of Hong Kong, who led the research team.
Hydrogen produced by low-carbon processes could play a key role in tackling climate change, by replacing polluting fossil fuels or feedstock chemicals. But storing the gas aboard hydrogen-powered vehicles has been a big challenge. A high-pressure hydrogen tank would have to be three or four times as large as a gasoline tank to provide an equivalent driving range, according to the US Department of Energy (DoE).
Porous materials can potentially store hydrogen in a more compact way. For instance, metal-organic frameworks (MOFs) have an open scaffold structure that can hold gas molecules, but they need to offer a high capacity: DoE targets suggest that hydrogen storage systems in vehicles should eventually carry at least 6.5% of their own massin hydrogen, with at least 50 g of hydrogen per liter of storage capacity. The latter volumetric goal is a key constraint on a car’s driving range.
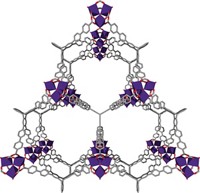
Stoddart’s team’s HOF beats both of these DoE targets—albeit only when kept very cold. Dubbed RP-H101, the material is built from Y-shaped triptycene molecules, whose three arms each carry an imidazole ring and a pair of carboxylic acids. During crystallization, hydrogen bonds form between carboxylic acids on one molecule and imidazoles on neighboring molecules, creating flat arrays of hexagons that stack into a 3D honeycomb.
Each of these hexagonal honeycomb cells has gaps that can accommodate more triptycene building blocks. These molecules assemble into six more 3D networks that interpenetrate the first, nesting in a phenomenon called catenation. This helps to make the crystals robust enough to resist common organic solvents and withstand temperatures up to 375 °C. It also creates two types of pores in the crystals that are just the right size for hydrogen molecules.
When storing and releasing hydrogen between –196 °C and 10,000 kPa and –113 °C and 500 kPa, RP-H101 had a gravimetric capacity of 9.3% and a volumetric capacity of about 53 g/L.
The catenated structure is “very beautiful” and has a “very high” hydrogen storage capacity, says Hiroyasu Furukawa of the University of California, Berkeley, who studies MOFs for gas storage and separation and was not involved in the work. But Furukawa thinks the material might struggle to perform well at the higher temperatures that are more realistic for vehicle storage systems.
“We still have a way to go for room temperature hydrogen storage applications,” says Ruihua Zhang of the University of Hong Kong, who co-led the experimental work. For now, says Stoddart, the material might be suitable for situations where cryogenic cooling systems can be used. He cofounded a company called H2MOF to develop hydrogen storage materials, and the new HOF will be further developed through an offshoot called H2Reserve.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter