Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Green Chemistry
A practical route to making ammonia from wastewater
Porous solid electrolyte boosts the efficiency of converting nitrates to ammonia
by Prachi Patel
August 14, 2024
| A version of this story appeared in
Volume 102, Issue 25

Wastewater is laced with nitrates that harm ecosystems and human health. Engineers have now designed a practical reactor based on a solid electrolyte to efficiently convert those nitrates into ammonia (Nat. Catal. 2024, DOI: 10.1038/s41929-024-01200-w). The method could treat wastewater while making ammonia production more sustainable.
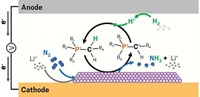
Ammonia-based fertilizers help feed the world. But the Haber-Bosch process to make ammonia results in 1.3% of global CO2 emissions. Synthesizing ammonia by the electrochemical reduction of nitrates, which could be powered by renewable electricity, is a greener alternative. In electrochemical devices, a catalyst at the anode oxidizes water to form hydrogen ions, which go to the cathode, where another catalyst reduces nitrate to ammonia.
But wastewater’s low nitrate concentrations mean much of the current ends up going toward producing hydrogen gas instead of ammonia. So researchers have previously added electrolyte solutions to conduct more hydrogen ions across the electrodes. “But in real wastewater treatment systems, you can’t add tons of electrolyte to convert nitrate to ammonia,” says Feng-Yang Chen, a chemical and biomolecular engineer at Rice University.
Chen, Haotian Wang, and colleagues designed a reactor that is split into three chambers using two electrodes. Each electrode is a ruthenium-embedded copper nanowire mesh to which the researchers attached an ion-conducting membrane. They packed the chamber between the electrodes with tiny beads of a porous polymer drenched with electrolyte solution.The new setup increased ionic conductivity and suppressed hydrogen gas formation. When the team fed simulated industrial wastewater with a typical 2,000 ppm nitrate concentration into the device, over 90% of electric current went toward ammonia production.
Previous systems suffer from much lower efficiencies at that concentration, says Xiao Su, a chemical and biomolecular engineering professor at the University of Illinois Urbana-Champaign who was not involved in the study. “The porous solid electrolyte is a clever conceptual advance and gives a significant performance improvement.” The long-term challenge will be to ensure that the process, with its use of novel catalysts and electrolytes, can compete in cost with the Haber-Bosch method.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter