Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biomaterials
Soft materials form surprising superstructures
Tunable hydrogel controls state of neural cells in culture
by Celia Henry Arnaud
October 4, 2018
| A version of this story appeared in
Volume 96, Issue 40

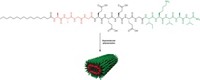
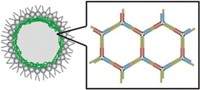
To mimic natural protein assemblies, Samuel I. Stupp and coworkers at Northwestern University set out to make a hydrogel that could undergo reversible changes in stiffness. They succeeded using peptide nanofibers—some with DNA attached. But the structures didn’t look quite as expected.
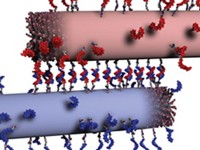
To the naked eye, the gels looked like normal hydrogels, but when the researchers imaged them with scanning electron microscopy, they found that instead of the normal meshlike fiber configuration, the gels consisted of long and thick bundles of fibers—superstructures—embedded in a matrix of individual fibers (Science 2018, DOI: 10.1126/science.aat6141). The self-assembly is reversible and controllable with the addition of other DNA strands.

The DNA-containing molecules start out randomly distributed throughout all the fibers, but eventually they concentrate in the superstructures. “A critical part is the dynamic nature of the system,” Stupp says. Molecules can exit from their original fiber and reorganize into the superstructures.
To understand the assembly mechanism, Stupp enlisted Northwestern colleague Erik Luijten, whose team ran monomer-level simulations. “The modeling predicted that molecules capable of strong interactions, even without DNA, could yield the same superstructures,” Luijten says. To test that, the researchers designed a second set of peptides that were held together by electrostatic interactions and formed similarly reversible superstructures. “In principle, any chemistry will be possible,” even inorganic systems, if conditions are right, Stupp says.
Stupp’s team used the hydrogels as artificial extracellular matrices to culture brain cells. When stiff superstructures formed, the cells switched from a healthy state to one that occurs during injury or neurological disease. The cells reverted to a healthy state when the rigid bundles went away. “We showed that the superstructure itself causes these changes in the cells,” Stupp says.
Ian Manners, an expert on supramolecular chemistry at the University of Bristol, says the work represents “important breakthroughs in the area of functional self-assembled materials.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter