Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.

Cathy Mulzer
Materials maven has powered up covalent organic frameworks and other polymers
by Matt Davenport
August 20, 2018 | APPEARED IN VOLUME 96, ISSUE 33
Coping with uncertainty is part of the job for any scientist. DowDuPont's Cathy Mulzer handles it with more aplomb than most. It seems almost as if she can look into the future and bring back instructions on how best to overcome obstacles facing her while developing advanced polymer materials in the lab.
Her Ph.D. adviser at Cornell University, William Dichtel, has an explanation that doesn't involve clairvoyance, however. "She's the perfect combination of intellectual firepower and outstanding attitude for research," says Dichtel, who is now at Northwestern University.
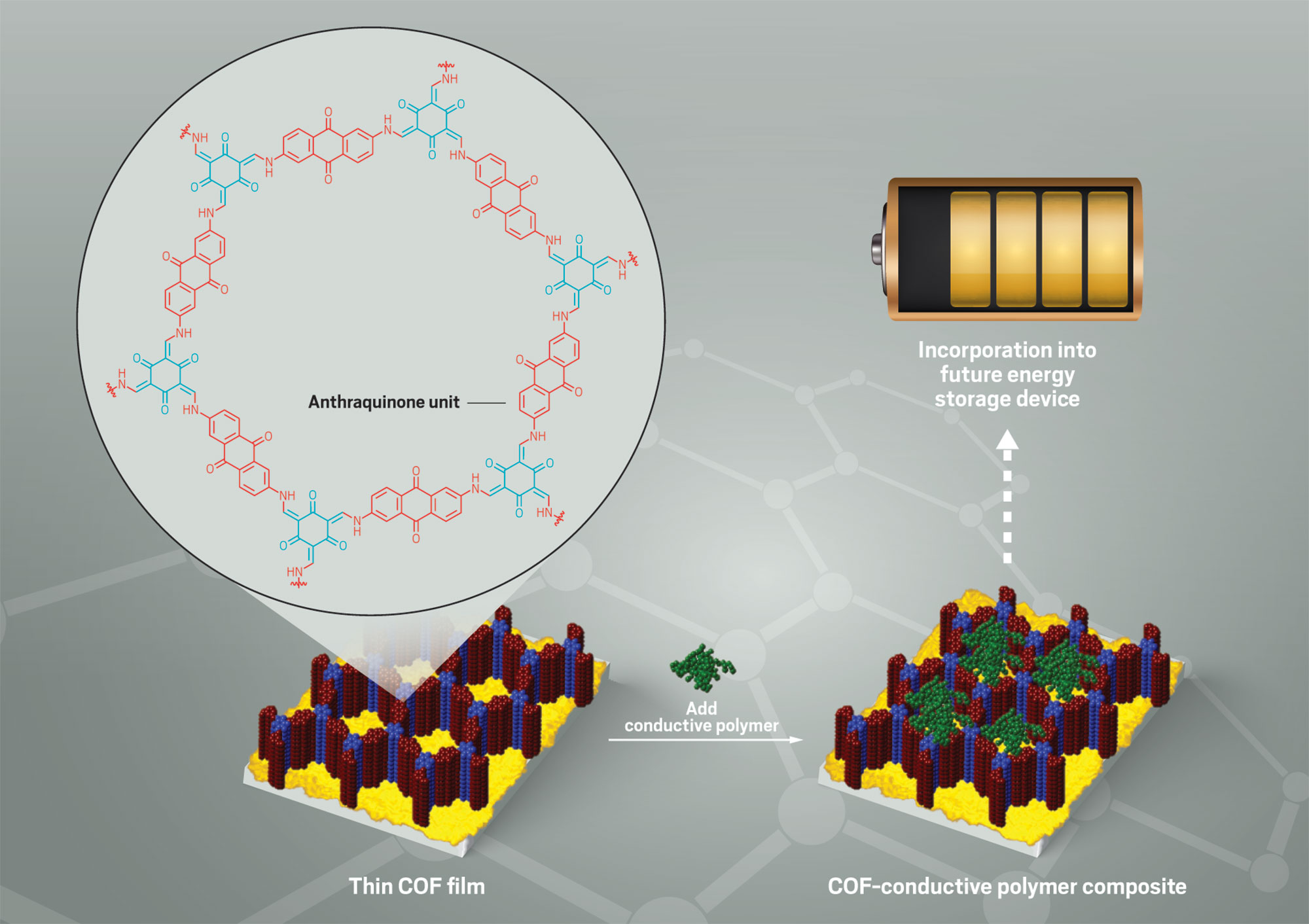
Mulzer's work in Dichtel's group is a prime example of these qualities. She investigated an emerging class of materials known as covalent organic frameworks, or COFs. COFs are porous crystalline polymers with insanely high surface areas, which make them attractive for things like catalysis and gas storage and separation.
Mulzer reasoned that because of their vast surface areas, COFs would also provide a wealth of parking spots for electrical charge carriers, making them attractive candidates for energy storage applications.
But existing COFs couldn't do the electrochemistry needed to shuttle those charges around—a necessity for energy storage. So step one for Mulzer and her colleagues was to synthesize a COF with redox active sites, which the team achieved with help from anthraquinone building blocks.
Making some of the first meaningful electrochemical measurements of COFs was really exciting, Mulzer says. The readings, however, revealed that the team had tapped into the electrochemical activity of only 3% of the polymer's available anthraquinone redox sites. "There's no way to sugarcoat it," she says. "It didn't work that well."
But that didn't deter Mulzer. To help charges get where they needed to go within the polymer, she learned to grow oriented thin films of the COF and then infiltrate its pores with conducting polymer. That 3% figure ballooned to 100%, she says, greatly boosting the COF's electrochemical performance.
While Mulzer was helping establish COFs as viable electronic materials with her persistence and chemical intuition, unbeknownst to her, she was also securing her future job. Both Dichtel and Cornell collaborator Héctor D. Abruña started recommending Mulzer to Dow recruiters two years before her graduation in May 2016.
Mulzer joined Dow that June, just over a year before the Dow-DuPont merger. She's not at liberty to discuss too many of the details of what she's working on for the company. However, her colleagues are eager to share that, in addition to developing next-generation polymer materials, she's been strengthening her workplace community.
Watch Mulzer speak at the American Chemical Society national meeting on Aug. 20 in Boston.
Vitals
Current affiliation: DowDuPont
Age: 29
Ph.D. alma mater: Cornell University
If I weren't a chemist, I would be: The owner of a bakery and ice cream shop. I love making desserts that make people smile.
If I were an element, I would be: Carbon. I am an organic chemist at my core, so I could not pick another element.
Latest TV show binge-watched: "Frasier" and "Chef's Table"
Walk-up song: "Shake and Vent," the Cornell chemistry department's parody of "Shake It Off" by Taylor Swift
Research at a glance
Credit: ACS Cent. Sci./Yang H. Ku/C&EN/Shutterstock
During graduate school, Mulzer and her coworkers synthesized the first covalent organic framework (COF) capable of interesting electrochemistry. She then beefed up the material's energy-storing performance by improving its crystalline ordering and combining it with a conductive polymer electrolyte.
Three key papers
"Superior Charge Storage and Power Density of a Conducting Polymer-Modified Covalent Organic Framework"
(ACS Cent. Sci. 2016, DOI: 10.1021/acscentsci.6b00220)
Electronic Materials
Cathy Mulzer
Materials maven has powered up covalent organic frameworks and other polymers
by Matt Davenport
August 19, 2018
| A version of this story appeared in
Volume 96, Issue 33


Vitals
Current affiliation: DowDuPont
Age: 29
Ph.D. alma mater: Cornell University
If I weren’t a chemist, I would be: The owner of a bakery and ice cream shop. I love making desserts that make people smile.
If I were an element, I would be: Carbon. I am an organic chemist at my core, so I could not pick another element.
Latest TV show binge-watched: “Frasier” and “Chef’s Table”
Walk-up song: “Shake and Vent,” the Cornell chemistry department’s parody of “Shake It Off” by Taylor Swift
Three key papers
“Superior Charge Storage and Power Density of a Conducting Polymer-Modified Covalent Organic Framework” (ACS Cent. Sci. 2016, DOI: 10.1021/acscentsci.6b00220)
“Moving Beyond Boron: The Emergence of New Linkage Chemistries in Covalent Organic Frameworks” (Macromolecules 2016, DOI: 10.1021/acs.macromol.6b00891)
“β-Ketoenamine-Linked Covalent Organic Frameworks Capable of Pseudocapacitive Energy Storage” (J. Am. Chem. Soc. 2013, DOI: 10.1021/ja409421d)
Coping with uncertainty is part of the job for any scientist. DowDuPont’s Cathy Mulzer handles it with more aplomb than most. It seems almost as if she can look into the future and bring back instructions on how best to overcome obstacles facing her while developing advanced polymer materials in the lab.
Her Ph.D. adviser at Cornell University, William Dichtel, has an explanation that doesn’t involve clairvoyance, however. “She’s the perfect combination of intellectual firepower and outstanding attitude for research,” says Dichtel, who is now at Northwestern University.
Mulzer’s work in Dichtel’s group is a prime example of these qualities. She investigated an emerging class of materials known as covalent organic frameworks, or COFs. COFs are porous crystalline polymers with insanely high surface areas, which make them attractive for things like catalysis and gas storage and separation.
Mulzer reasoned that because of their vast surface areas, COFs would also provide a wealth of parking spots for electrical charge carriers, making them attractive candidates for energy storage applications.
But existing COFs couldn’t do the electrochemistry needed to shuttle those charges around—a necessity for energy storage. So step one for Mulzer and her colleagues was to synthesize a COF with redox active sites, which the team achieved with help from anthraquinone building blocks.
Making some of the first meaningful electrochemical measurements of COFs was really exciting, Mulzer says. The readings, however, revealed that the team had tapped into the electrochemical activity of only 3% of the polymer’s available anthraquinone redox sites. “There’s no way to sugarcoat it,” she says. “It didn’t work that well.”
But that didn’t deter Mulzer. To help charges get where they needed to go within the polymer, she learned to grow oriented thin films of the COF and then infiltrate its pores with conducting polymer. That 3% figure ballooned to 100%, she says, greatly boosting the COF’s electrochemical performance.
While Mulzer was helping establish COFs as viable electronic materials with her persistence and chemical intuition, unbeknownst to her, she was also securing her future job. Both Dichtel and Cornell collaborator Héctor D. Abruña started recommending Mulzer to Dow recruiters two years before her graduation in May 2016.
Mulzer joined Dow that June, just over a year before the Dow-DuPont merger. She’s not at liberty to discuss too many of the details of what she’s working on for the company. However, her colleagues are eager to share that, in addition to developing next-generation polymer materials, she’s been strengthening her workplace community.
















Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter