Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Metal-Organic Frameworks
MOFs neutralize nerve agents without needing liquid water
Composites made of MOFs and solid bases mediate hydrolysis reactions using water from the air
by Mitch Jacoby
December 30, 2019
| A version of this story appeared in
Volume 98, Issue 1

By combining a porous metal organic framework (MOF) compound and a solid base, researchers have made a hybrid material that can be applied to textiles and used to deactivate chemical warfare agents under ambient conditions (J. Am. Chem. Soc. 2019, DOI: 10.1021/jacs.9b11172). The finding may lead to new types of fabrics for gas-mask filters and protective suits that are more effective than today’s variety at trapping and neutralizing the poisons.
Chemical weapons based on organophosphate nerve agents such as soman, also known as GD, and a chemically related phosphonothioate known as VX, rank among the most toxic compounds known. Gas masks and other protective equipment generally use activated carbon and metal oxides to protect soldiers and others from these chemicals.
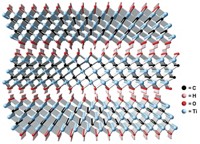
In recent years, researchers have shown that some MOFs, which are porous materials composed of metal ions bridged by organic linking groups, have the potential to do a better job than the standard materials when it comes to trapping and neutralizing nerve agents. To use MOFs for personal protection, researchers have been studying ways of incorporating these materials into gas-mask filters and fabrics.
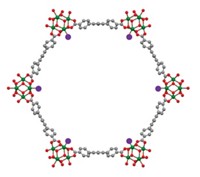
Although these earlier studies show that MOFs can catalytically hydrolyze nerve agents, the neutralization reactions typically require bulk alkaline solutions containing volatile, toxic bases. That requirement makes MOFs better suited to facilities for large-scale decontamination than to personal protective gear. In the new study, Zhijie Chen and Omar K. Farha of Northwestern University and coworkers report that treating zirconium-based MOFs with linear polyethylenimine (PEI), a nonvolatile and relatively benign solid base, results in a tough composite that circumvents the need for liquid water. The composites, formed by mixing MOF and PEI powders, are effective because the porous materials can store enough water from ambient humidity to mediate hydrolysis reactions.
The team carried out a series of tests, evaluating the composites’ effectiveness in hydrolyzing GD, VX, and a related compound known as DMNP. They conducted tests on neat MOF-PEI composites and on composites coated onto inexpensive commercial fibers and large textile samples. In general, they found that when the composites were used at roughly 50% relative humidity, they worked nearly as well as or better than other MOFs did in liquid alkaline solution. In addition, the composites remained active after 100 days of unprotected storage and after being exposed to various contaminants.
“This study significantly advances the application of MOFs to catalytic destruction of nerve agents,” says University of South Carolina’s Natalia B. Shustova, a specialist in porous materials. These composites, grafted onto textiles, “demonstrate remarkable robustness,” she says, standing up to contaminants including CO2, sweat, and vehicle emissions. These results, she says, suggest a path for commercializing MOFs for “protective garments against chemical warfare agents.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter