Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
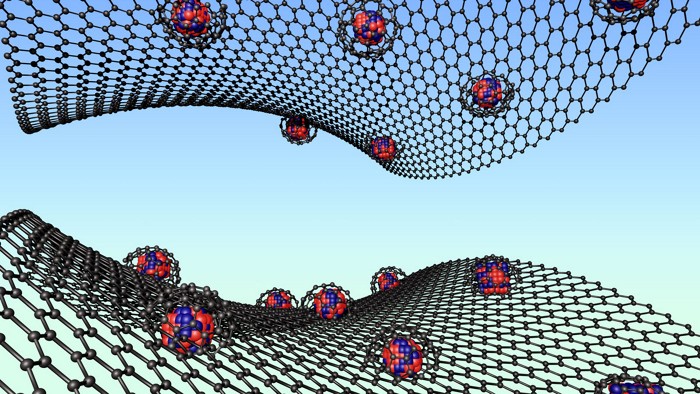
Metal-Organic Frameworks
New coating technique protects MOF nanoparticles and improves drug delivery properties
Simple shelf-life-improving process also yields slow-release drug carriers
by Fernando Gomollón-Bel, special to C&EN
September 9, 2021

Researchers at the University of Cambridge have reported a simple chemical process that increases the stability of metal-organic frameworks—porous networks of metal ions and organic molecules—and improves their ability to gradually release drugs over several days (J. Am. Chem. Soc. 2021, DOI: 10.1021/jacs.1c03943).
Metal-organic framework nanoparticles (nanoMOFs) are of interest in drug delivery because their honeycomb-like molecular pores can adsorb and later release drug molecules in a controlled way. But when nanoMOFs are dissolved in buffer solutions, they can often become unstable. Specifically, the phosphate ions in buffers, which are ubiquitous in pharmaceutical applications, tend to attack the metallic centers in a nanoMOF’s structure and eventually break down its network. “Most buffers are kryptonite to MOFs,” explains David Fairén-Jiménez, lead author of the study.
However, the researchers turned this to their advantage by coating nanoMOFs with a phosphate that had a polyethylene glycol (PEG) chain attached to it. Previous approaches had covered MOFs with PEG, but the phosphate group used in this approach provides particularly strong binding to nanoMOFs’ metal centers. Because the phosphate group binds so tightly, the resulting products are more stable than previous coated nanoMOF preparations, says Tendai Gadzikwa, an expert in MOFs at Kansas State University who was not involved in the study.
The synthesis takes only two steps: formation of the MOF and functionalization with the phosphate. The method’s simplicity offers an interesting platform for future enhancements of nanoMOFs, says Omar Yaghi, a MOF pioneer at the University of California, Berkeley.
The resulting coating is both biocompatible and protective, preventing the nanoMOF from breaking apart in solution.
“This is a major advantage to control the release of drugs,” says Yaghi.
As an added bonus, whereas other nanoMOFs quickly release their contents in short amounts of time—usually less than 48 hours—the new polymer-coated structures gradually leaked a model drug into solution over 12 days. This sustained release could be a boon in, for example, diabetes treatments, in which encapsulated insulin needs to be delivered over long periods of time, Fairén-Jiménez explains.
Furthermore, the coating allows for the dry storage of nanoMOFs, useful for new pharmaceutical formulations. Traditionally, nanoMOFs are stored in solution, which leads to destabilization and decomposition of the nanoparticles, Yaghi explains. And if non-coated nanoMOF dispersions are dried the particles clump up and form aggregates, which makes redissolving them complicated, increases their toxicity, and often reduces their ability to adsorb other molecules. However, freeze-drying the coated nanoMOFs yields durable powders in which nanoMOFs maintain their porous structure and thus maintain their ability to carry drugs. The team showed the method improved the stability of five different nanoMOFs.
Gadzikwa considers this a major breakthrough: Now, “MOFs can be stored in the more stable solid form, then easily redispersed as needed,” she says. Beyond nanomedicine, the new method could provide advantages in catalysis, gas storage, and other applications of MOFs.
The Cambridge team also evaluated the toxicity of the new materials, which seem less harmful than their non-coated counterparts. “This is just a first step,” explains Fairén-Jiménez. “We want to keep studying polymer-coated MOFs and develop new solutions to tackle hard-to-treat cancers,” he adds. In fact, Fairén Jiménez has filed a patent on this discovery and recently cofounded the start-up Vector Bioscience Cambridge, which will focus on developing innovative and selective cancer treatments.
To that end, additional chemical modifications will enable more targeted delivery. Yaghi agrees that further research is needed, yet these preliminary results provide promise for better drug delivery and fruitful use in the pharmaceutical industry.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter