Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Polymers
Thermoset plastic made from wood waste catalyzes its own degradation
Scientists design tough cross-linked material that can be chemically recycled under mild conditions
by Brianna Barbu
April 12, 2024
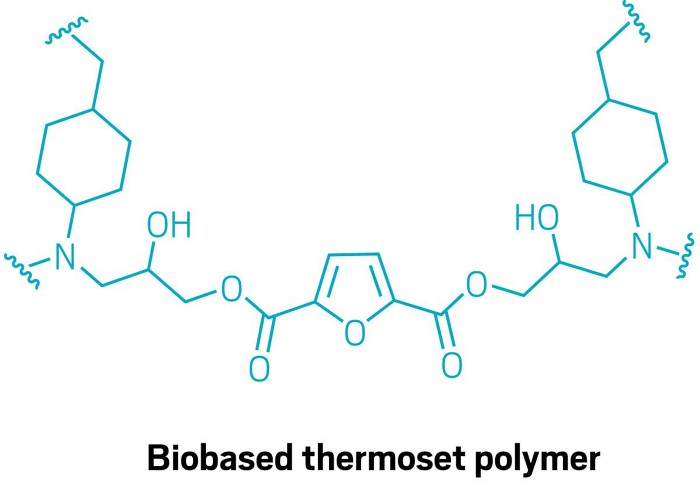
A future when tough polymer resins and composites such as those found in wind turbine blades could be made entirely from plant-based, recyclable components just got a little closer. Researchers led by Katalin Barta at the University of Graz have designed a new thermoset polymer, derived from wood, that can be broken down in methanol with no added catalyst (Science 2024, DOI: 10.1126/science.adj9989).
Thermoset polymers have cross-linked networks that make them exceptionally tough and useful materials. But they are also practically impossible to break down or recycle. And epoxy-amine resins, one of the most common thermoset plastics, are typically made using bisphenol A (BPA), which is an endocrine disruptor.
Plenty of researchers have tried making degradable thermoset plastics by incorporating functional groups whose bonds can be severed by a catalyst or other external trigger. Barta and coworkers designed their new biobased epoxy-amine polymer similarly, with easily cleaved ester groups in the polymer backbone. But the polymer turned out not to need an external catalyst to break it down. “The fact that it catalyzes its own degradation was definitely serendipity,” Barta says. “We didn’t hope for such a wonderful effect.”
Research in Barta’s group focuses on developing useful chemical building blocks from plant-based feedstocks such as lignin, a waste product from papermaking. To make the recyclable resin, the researchers combined lignin-derived 4,4′-methylenebiscyclohexanamine (MBCA) with an epoxy component made from 2,5-furandicarboxylic acid and glycidol, both of which also come from plant-based sources.
The polymer has physical properties matching those of petrochemical-based materials, including its glass transition temperature. It’s also resistant to most solvents, including acidic and basic aqueous solutions, acetone, and dichloromethane. But Barta and Xianyuan Wu, who worked on the project as a graduate student, were surprised to discover that the material started breaking down and dissolving when soaked in methanol.
The researchers determined that hydrogen-bonding interactions between the ester, amine, and methanol activate the carbonyl and initiate a transesterification process that breaks up the polymer network. It takes about 48 h at 70 °C for the polymer to degrade to the point at which the methyl ester of 2,5-furandicarboxylic acid can be recovered in 90% yield. The researchers could also recover the MBCA and glycidol pieces, so every component of the polymer is recyclable to some degree.
Eugene Y.-X. Chen, a polymer chemist at Colorado State University who was not involved in the work, calls it “a superb example of demonstrating that biobased polymers, even in a robust cross-linked network form, can also exhibit recyclability advantages.” He says it was “truly remarkable” to see an epoxy-amine resin that can be depolymerized under relatively mild conditions with no external catalyst.
The team is still working to optimize the recycling process and expand the scope of recyclable epoxy-amine polymers, but “there is definitely a potential there,” Barta says. She hopes to start looking into industrial partnerships and applications for the material soon.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter