Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Pharmaceuticals
Click chemistry sees first use in humans
Targeting mechanism could help to avoid side effects of powerful anticancer drug
by Mark Peplow, special to C&EN
October 21, 2020

A therapy that relies on click chemistry to target a powerful cancer drug at tumor cells, sparing healthy ones, has entered Phase 1 clinical trials. It marks the first time that a click chemistry reaction has been carried out inside a patient’s body, according to Shasqi, the San Francisco–based biotech firm that developed the therapy.
The work marks a milestone in bioorthogonal chemistry, which relies on reactions that can run inside living organisms without disrupting biochemical processes. So-called click reactions have become a mainstay of this approach because they occur exclusively between two synthetic molecules, rapidly and irreversibly clicking them together. Researchers already use bioorthogonal chemistry to tag biomolecules with imaging agents, such as fluorescent probes; and it has helped to manufacture targeted therapeutics, such as antibody-drug conjugates, that have subsequently entered clinical trials.
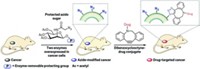
Shasqi’s cancer therapy exploits one of the fastest click reactions, a Diels-Alder cycloaddition between a tetrazine and a trans-cyclooctene (TCO). “The chemistry doesn’t care what’s inside the cell, and the cell doesn’t care that there’s some strange chemistry going on inside it,” says Maksim Royzen at the University of Albany, who collaborates with Shasqi and was one of the researchers who developed the tetrazine-TCO reaction (J. Am. Chem. Soc. 2008, DOI: 10.1021/ja8053805).
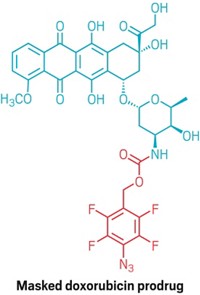
The therapy involves two key components. The first is a sodium hyaluronate biopolymer that is modified to include tetrazine groups. The second is a cancer drug called doxorubicin that is connected to a TCO unit. Doxorubicin is used against more than a dozen different cancers, including breast, ovarian, and liver cancers, but it can cause severe side effects that interrupt treatments. In contrast, a recent study in mice by Royzen and Shasqi, published on the preprint server ChemRxiv, suggests that doxorubicin-TCO is roughly 80 times less toxic than the parent drug (ChemRxiv 2020, DOI: 10.26434/chemrxiv.13087715.v1). The study has not yet been peer reviewed.
During the trial, the biopolymer is injected into a cancer patient’s tumor. That patient then receives five daily infusions of doxorubicin-TCO, which circulates through the body until it meets the tetrazine-modified biopolymer. At that point, the click reaction brings the tetrazine and TCO together, triggering a rearrangement that frees doxorubicin right next to the tumor cells. This rearrangement step, involving a carbamate cleavage, had previously been used to release a drug molecule from a tetrazine-TCO adduct, in research from a team led by Marc S. Robillard at Tagworks Pharmaceuticals (Angew. Chem. Int. Ed. 2013, DOI: 10.1002/anie.201305969).
As well as reducing side effects from the drug, this approach should also increase the local concentration of doxorubicin far beyond what would normally be possible in a patient. “Click chemistry is at the core of what we do because it enables us to differentiate a particular location from the rest of the body,” says José M. Mejía Oneto, founder and CEO of Shasqi. The company announced on Oct. 14 that it has dosed the trial’s first two patients over the previous two months.
Oneto hopes the treatment could be particularly beneficial against a rare type of cancer called soft tissue sarcoma. These cancer cells lack suitable biomarkers that could be targeted by antibody-drug conjugates, another strategy being used to guide cancer therapies.
Shasqi’s previous experiments on mice with sarcoma grafts showed that they could tolerate doses of doxorubicin-TCO six times as high as those of the parent drug without significant side effects. In the study, which has yet to undergo peer review, the treatment increased mouse survival by 63% (bioRxiv 2020, DOI: 10.1101/2020.10.13.337899).
“It’s a very exciting advance,” says Jason S. Lewis at the Memorial Sloan Kettering Cancer Center, whose research involves bioorthogonal chemistry and who is not involved in Shasqi’s work. “I’m hoping that they get at least an equivalent response in patients and hopefully a reduction in side effects.”
Lewis has developed an imaging agent that uses the tetrazine-TCO reaction to unite a copper-64 radioisotope and a cancer-targeting antibody inside patients. This should tag cancer cells with the radioisotope so that they can be located by positron emission tomography. He hopes to begin a clinical trial of the agent, using an antibody owned by German biotech firm BioNTech, early next year.
“I think these two are really in the vanguard,” says Carolyn R. Bertozzi at Stanford University, a pioneer of bioorthogonal chemistry, who is also a consultant to Shasqi. “If Shasqi succeeds, and if Jason succeeds, that’s really gonna blow the door open, because it derisks the whole idea of doing this in vivo chemistry.”
Shasqi’s strategy—which it calls Click Activated Protodrugs Against Cancer (CAPAC)—is currently only suitable for solid tumors that can be readily or safely injected. But Shasqi’s research suggests that CAPAC could be used to deliver other cancer drugs, including paclitaxel, etoposide, and gemcitabine.
Shasqi hopes to recruit a total of 40 patients to its current trial, which it expects to complete in 2021.
UPDATE:
This story was updated on Nov. 4, 2020, to clarify that the rearrangement step that releases doxorubicin was inspired by work from a team led by Marc S. Robillard at Tagworks Pharmaceuticals.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter