Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
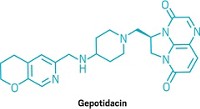
Antibiotics
New antibiotics are hard to come by. Red tape is making the problem worse
What happens when the reward for making a new antibiotic is bankruptcy?
by Rowan Walrath
May 31, 2024
| A version of this story appeared in
Volume 102, Issue 17

It’s a story 30-odd years in the making. The tale begins in the 1990s, when Roche had an anti-infectives unit. Back then, plenty of pharmaceutical companies had scientists working on novel antibiotics, inspired financially by the 50 years of successful antimicrobial development that had preceded them and morally by the rise of drug-resistant bacteria.
Roche scientists had the idea to create a systemic β-lactam antibiotic for methicillin-resistant Staphylococcus aureus, better known as MRSA. It was the early days of antimicrobial resistance—or at least the appreciation of it—and MRSA appeared to be the greatest threat among drug-resistant bacteria.
β-lactams work by binding to penicillin- binding proteins (PBPs), enzymes that are crucial to bacteria’s ability to form cell walls. Binding PBPs stops those walls from coming together. But MRSA was near impossible to drug with a β-lactam because MRSA has a gene called mecA. That gene produces a specific kind of PBP called PBP2a, which reacts with early-generation β-lactam drugs significantly slower than native PBPs do, allowing bacterial cells to keep their cell walls together (Org. Process Res. Dev. 2017, DOI: 10.1021/acs.oprd.7b00143). Other drug-resistant bacteria often have similarly tricky variants of PBP.
Roche’s drug, ceftobiprole medocaril sodium (ceftobiprole), breaks the mold for β-lactams. It has a strong affinity for PBP2a in MRSA, PBP2x in penicillin- resistant Streptococcous pneumoniae, PBP1a and PBP2-4 in Escherichia coli, PBP1a-b and PBP3-4 in Pseudomonas aeruginosa, and PBP5 in Enterococcus faecalis.
So work on the antibiotic continued, and when Roche spun out its infectious diseases and dermatological units into a new company called Basilea Pharmaceutica in 2000, the program went with it.

A few years later, Basilea struck a partnership with pharma giant Johnson & Johnson, which would develop, commercialize, and manufacture the drug. But manufacturing slip-ups and a shifting regulatory landscape forced Basilea—whose partnership with Johnson & Johnson ended in 2010—to reengineer its data package multiple times, including by running another large clinical trial on top of the studies it had already conducted.
“Initially, it was all positive,” says Basilea chief medical officer Marc Engelhardt. “In 2008, there was a conclusion that this drug seemed to provide benefit for patients, and there was no safety concern. Then, this downward spiral developed.”


First, the US Food and Drug Administration questioned the integrity of the trial on which the new drug application was based, citing manufacturing issues by Basilea’s then partner Johnson & Johnson. Then, in 2014, the agency asked Basilea to add Phase 3 data to its submission package, in line with recently changed requirements for community- and hospital-acquired pneumonia medications. US plans were put on hold. Meanwhile, Basilea was steadily accumulating approvals from regulators in other regions, including Europe, Latin America, China, and Canada.
Large-scale clinical trials can cost companies hundreds of millions of dollars and take years to complete. Basilea wouldn’t be able to fund another Phase 3 without the help of a partner, executives said. Another division of the US government, the Biomedical Advanced Research and Development Authority (BARDA), ultimately stepped in and granted Basilea about $134 million starting in 2016. Basilea executives estimate BARDA covered about 70% of the project’s costs.

This year, on April 3, the FDA finally approved ceftobiprole, marketed under the name Zevtera, to tackle susceptible isolates of MRSA and a slew of other bacteria—Streptococcus pyogenes, Klebsiella pneumoniae, Haemophilus influenzae, Haemophilus parainfluenzae, and E. coli—that cause bloodstream and skin infections as well as pneumonia.
“Having a β-lactam for MRSA was a big deal, and they had the best one, in my opinion,” says Ryan Cirz, an antibiotic developer and CEO of biotech start-up Revagenix. “I’m happy they finally got it to market, but my God, that program’s been going on for like 30 years.”
Zevtera is one of three antibiotics to gain FDA approval for humans so far this year, and the only systemic one. The other two are for urinary tract infections (UTIs): Utility Therapeutics’ Pivya, for uncomplicated UTIs; and Allecra Therapeutics’ Exblifep, for complicated, or drug-resistant, UTIs.
But outside of 2024, the US has approved few new antibiotics in recent years. Only 17 new systemic antibiotics and one related biologic netted approval between 2010 and May 2021 (Ann. Pharmacother. 2022, DOI: 10.1177/10600280211031390). Experts worry that even that number could represent a peak. These approvals were decades in the making, and a labyrinth of scientific, financial, and regulatory challenges are sending today’s antibiotic developers fleeing.
A resistant market
There’s a unique web of disincentives for new antibiotic development. Bacteria are constantly morphing to better evade the drugs that are designed to kill them, changing the structures of their membranes, emitting enzymes that inactivate certain antibiotics, and developing efflux pumps that spew out the drugs that do manage to get inside their cells. That shape-shifting means first-line treatments like penicillin and methicillin are no longer as effective, necessitating new medicines.
But these new medicines are meant to be used only as a last resort, after all other options have been exhausted. To make matters worse, physicians tend to prefer generic antibiotics that have been on the market for decades—even when those drugs aren’t working—because they’re cheaper and broadly known to be safe. A recent National Institutes of Health–led study found that from 2016 to 2021, more than 40% of US hospital patients with drug-resistant bacterial infections were treated exclusively with traditional antibiotics, even when newer options, including next-generation antibiotics for gram-negative infections, were available (Ann. Intern. Med. 2024, DOI: 10.7326/M23-2309).
Rising resistance
The timeline from antibiotic deployment to when resistance was first observed, for multiple antibiotics
Source: Science 2001, DOI: 10.1126/science.293.5536.1786
For investors, these dynamics mean fewer sales opportunities and, consequently, no commercial market for new antibiotics.
“Most investors have blinders on and just view this as a dysfunctional market,” says Henry Skinner, CEO of the institutional investment firm AMR Action Fund, which backs companies fighting antimicrobial resistance (AMR) with late-stage assets. “Putting aside all the other challenges, which are real, how do you break through the perception that this is just a field you cannot touch as an investor?”
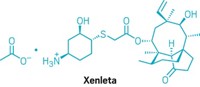
The AMR Action Fund is among just a handful of private investment firms that back companies developing novel antibiotics. One of its investments was in Utility Therapeutics, maker of the uncomplicated UTI drug Pivya. Pivya (pivmecillinam) is a prodrug that, when swallowed, hydrolyzes to become the β-lactam antibacterial drug mecillinam, also called amdinocillin. Mecillinam stops the biosynthesis of bacterial cell walls by targeting PBP2 and kills E. coli, Proteus mirabilis, and Staphylococcus saprophyticus.
Like Zevtera, Pivya had been used in Europe for quite a while before it was approved in the US in April. Nordic physicians in particular have steadily prescribed it for around 40 years for uncomplicated UTIs, with low rates of resistance that Utility cofounder Morten Sommer ascribes to collateral sensitivity interactions with other drugs (Nat. Commun. 2019, DOI: 10.1038/s41467-019-08529-y)—when a bacterium becomes resistant to one antibiotic, it becomes more sensitive to Pivya and vice versa—and high fitness cost of resistance mutations (Nat. Rev. Microbiol. 2017, DOI: 10.1038/nrmicro.2017.75). Pivmecillinam also targets a part of the microbe different from the targets of its peers in the penicillin family.
Utility hopes to launch Pivya in the US next year, and to do it at a relatively low cost. The company effectively has only one full-time employee, CEO Thomas Hadley. The rest are consultants and contractors. Hadley plans to use the same factories that have been manufacturing Pivya in Europe and set up a supply chain linking them to the US.

Despite the company’s low overhead and Pivya’s long history of safety and effectiveness, Utility didn’t gain much traction with investors. The AMR Action Fund was its only institutional venture investor.
“Let’s just say we weren’t the darling of Wall Street,” Hadley says. “Given that antibiotics are not the sweet spot for funding these days, we had to be smart about how we spent money and how we got the product approved.”
That’s not uncommon for companies making anti-infectives. Venture capital and private equity firms seek high returns on investment to satisfy their own investors. Since sales for antibiotics need to be limited—otherwise, the rate of resistance will speed up—there’s essentially no business case to be made. Median annual sales of antibiotics in the first market they were launched in, usually the US, were just $16.2 million between 2010 and 2019 (Clin. Infect. Dis. 2021, DOI: 10.1093/cid/ciab612).
Basilea has multiple antibacterial and antifungal products it’s working to commercialize. On the whole, that portfolio, including Zevtera, could realistically generate peak sales of $500 million to $1 billion per year, Engelhardt estimates. To maximize profit, the company doesn’t invest in its own commercial infrastructure but relies on partners instead, Engelhardt says. Even then, no one expects blockbuster returns.
“It’s not going to be $10 billion like oncology or weight loss,” he says.
And that revenue won’t come flooding in right after approval. In biotech, there’s a concept called the valley of death. It marks the stretch of time between when a firm discovers promising science and when that science is de-risked enough, usually with human data, that the firm can raise money to advance it. In antibiotics, there’s a second valley of death that takes place after regulatory approval and before the company can sell enough of the drug to become financially solvent.
Revagenix’s Cirz experienced this firsthand. His previous company, Achaogen, was a trailblazer for new antibiotics targeting gram-negative bacteria in the early 2000s. The FDA approved its complicated UTI drug, Zemdri, in June 2018. Ten months later, Achaogen filed for bankruptcy. Cirz lost his life savings.
“[Making Zemdri] cost Achaogen a fortune, because we had to kind of bulldoze the path,” Cirz says. “By the time we got to the end of the road, it had cost so much money, finding an exit for an appropriate value was not going to be possible. People were starting to figure out, ‘Hey, these products are probably not going to make any money.’ But it was the early days of that.”
Soon other antibiotic developers began to drop off. A few months after Achaogen did, in December 2019, Melinta Therapeutics filed for bankruptcy, despite having four FDA-approved antibiotics in its portfolio at the time. (Melinta ultimately entered a restructuring agreement with the investment firm Deerfield Management and has since somewhat recovered.) As of 2022, of the 12 antibiotic companies that had gone public in the past 10 years, just five were still active, per a report from the trade group Biotechnology Innovation Organization (BIO). The other seven were acquired at fire-sale valuations, reverse-merged with other companies, or simply wound down.
Efforts to fix this broken market have stalled, in part because of a shift in attention and funds to address the COVID-19 pandemic. Meanwhile, the problem of antibiotic resistance has only grown. Three-quarters of people hospitalized with COVID-19 between January 2020 and March 2023 were treated with antibiotics “just in case” they might help, even though only about 8% of those patients actually had bacterial infections, the World Health Organization (WHO) reported in April.
“It’s a matter of getting new mechanisms of action to keep up with the arms race that we have with the bacteria,” says Utility’s Sommer, who works as a professor and scientific director at the Technical University of Denmark and has cofounded numerous other biotech firms. “The issue is that the golden age of antibiotic discovery started 60 years ago. At some point, people felt that resistance was not an issue any longer. Infectious disease had been solved, and innovation has stopped.”
An ancient problem
Most antibiotics started out as natural products, not made from scratch by chemists. Microbes have been evolving defense mechanisms since time immemorial to use against one another, battling for the highest spot in the food chain.
Penicillin, which ushered in the modern era of medicine, was famously discovered when the bacteriologist Alexander Fleming noticed mold secreting a self-defense mechanism that could kill bacteria. Penicillin was purified in the 1930s and deployed in the 1940s, gaining notoriety as it saved the lives of World War II soldiers whose battlefield wounds had become infected.
More classes of antibiotics were discovered in a similar fashion. Tetracyclines were first isolated from Streptomyces aureofaciens bacteria in the 1940s. The following decade, cephalosporins were taken from a fungus found in sewage off the coast of Sardinia. And so it’s gone, with the major exceptions of sulfonamides, quinolones, oxazolidinones, and recently oxepanoprolinamides, which are all completely synthetic (Angew. Chem. 2006, DOI: 10.1002/anie.200600350).
Because antibiotics are as ancient as bacteria themselves, so are bacterial resistance mechanisms. Clindamycin-resistant bacteria, for instance, stop the drug from binding to their ribosomes by adding two methyl groups to one of the ribosome’s nucleotides. Some multidrug-resistant bacteria add on extra efflux pumps that spit out the antibiotics that make their way inside. Many change the shape of proteins that antibiotics would otherwise bind to in their cell walls—that’s the mechanism by which MRSA evades methicillin. Gram-negative bacteria currently mark the greatest threat to antibiotics, because each has an additional cell wall that’s hard to penetrate.
Push and pull
The US has some tools in its arsenal to speed up antibiotic approval. All three antibiotics that the FDA approved this year were considered qualified infectious disease products (QIDPs). The QIDP designation enables drug developers to request that the FDA fast-track their compounds based on Phase 2 studies rather than Phase 3 and give the submissions priority review, which brings the target time period to an FDA decision down from 10 months to 6. QIDP also tacks on an additional 5 years of exclusivity, during which the approved drug won’t have any new generic competitors.
Advertisement
QIDP designation is a by-product of the 2012 GAIN Act, short for Generate Antibiotics Incentives Now. But it’s “fallen short of its goals,” as Harvard Medical School’s Jonathan Darrow and Aaron Kesselheim wrote in 2020, pointing to a mismatch between clinical need and program requirements, as well as language that incentivizes modifying existing drugs rather than creating new ones (Open Forum Infect. Dis. 2020, DOI: 10.1093/ofid/ofaa001).
“I think there’s a recognition that the process isn’t a complete cure-all, no pun intended, for what’s going on,” says former FDA lawyer Michael Varrone, who took a private-sector job at the law firm Sidley Austin in April.
Congress is currently considering another bill, the Pioneering Antimicrobial Subscriptions to End Upsurging Resistance (PASTEUR) Act, which would authorize the US Department of Health and Human Services to set up subscription plans for drugs designated as critical-need antimicrobials. It’s like Netflix for antibiotics: the government pays for access, and providers use what they need. The plan would ensure drugmakers get paid for new antibiotics regardless of sales volume.
But the PASTEUR Act has been introduced three times since 2020 and has never made it out of committee. It’s not feasible for drugmakers to wait around for its passage.
“We have been waiting for legislation for 15 years,” Cirz says. “It’s a fool’s errand to assume that in your model.”
If the US market seems broken, consider this: it is still the most attractive market for all new pharmaceuticals, including antibiotics, because companies are able to price drugs higher in the US than in other parts of the world.
Low- and middle-income countries fare much worse. Last-line treatments called reserve antibiotics were registered with local health authorities in an average of two out of 102 low- and middle-income countries as of 2021, according to a report from the Access to Medicine Foundation. And those countries bear the highest burden of antibiotic resistance, with resistance- related mortality 1.5 times as high as in high-income nations (J. Antimicrob. Chemother. 2023, DOI: 10.1093/jac/dkad291). All told, an estimated 4.95 million deaths were associated with drug-resistant bacteria worldwide in 2019 (Lancet 2022, DOI: 10.1016/S0140-6736(21)02724-0).
Some high-income countries are advancing plans along the lines of the PASTEUR Act called “pull incentives.” The UK recently wrapped up a subscription pilot program through the National Health Service and is in the process of implementing a permanent version. It also recently announced a $165 million public-private initiative to develop new diagnostics and treatments.
The European Union is likewise examining options that could include a subscription model or a voucher for longer exclusivity periods, but like the US, it is moving slowly. The European Parliament likely won’t come up with a formal position until next year, following elections, according to Frederic Peyrane, a chemist by training and financial consultant who works with the BEAM Alliance, a collection of biotech companies involved in AMR research in Europe. Meanwhile, 60% of BEAM’s members have less than a year before they run out of money.
Beyond funding, there’s also movement underway to streamline regulatory processes internationally. Ideally, such harmonization would make it cheaper for drug developers to launch internationally and make it so people in low-resource areas would get access to the same medicines people in the US and its high-income peers enjoy.
Such a process could soon become a reality. In May, the WHO designated the FDA, plus the European Medicines Regulatory Network and its member agencies, as WHO-Listed Authorities (WLAs), meaning other countries can look to them for registration and marketing authorization, clinical trial oversight, and other functions designed to ensure new medicines are safe and effective. The European and US agencies join Switzerland’s Swissmedic, South Korea’s Ministry of Food and Drug Safety, and Singapore’s Health Sciences Authority, which became WLAs in October.
“At the end of the day, I think innovators don’t really care about the mechanism that will be put in place, provided that it’s big enough, it’s predictable enough, and it just is basically workable. What we want is to be able to race forth,” Peyrane says. “Is the ecosystem dry? Yes. Do we need something to fix it? Yes, urgently. If [antibiotic companies] die, the pipeline will collapse, and the skills to develop new antimicrobials will vanish.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter